Acetaldehyde
| Acetaldehyde | |
|---|---|
 |
 |
 |
 |
|
Acetaldehyde
|
|
|
Ethanal
|
|
|
Other names
Acetic Aldehyde
Ethyl Aldehyde[1] |
|
| Identifiers | |
| CAS number | 75-07-0 |
| PubChem | 177 |
| ChemSpider | 172 |
| EC number | 200-836-8 |
| RTECS number | AB1925000 |
|
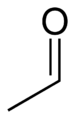
SMILES
O=CC
|
|
|
InChI
InChI=1/C2H4O/c1-2-3/h2H,1H3
Key: IKHGUXGNUITLKF-UHFFFAOYAB |
|
| Properties | |
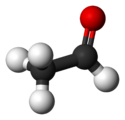
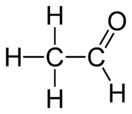
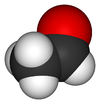
| Molecular formula | C2H4O |
| Molar mass | 44.05 g mol−1 |
| Appearance | Colourless liquid Pungent, fruity odor |
| Density | 0.788 g cm−3 |
| Melting point |
−123.5 °C, 150 K, -190 °F |
| Boiling point |
20.2 °C, 293 K, 68 °F |
| Solubility in water | soluble in all proportions |
| Viscosity | ~0.215 at 20 °C |
| Structure | |
| Molecular shape | trigonal planar (sp²) at C1 tetrahedral (sp³) at C2 |
| Dipole moment | 2.7 D |
| Hazards | |
| EU classification | Very flammable (F+) Harmful (Xn) Carc. Cat. 3 |
| R-phrases | R12 R36/37 R40 |
| S-phrases | (S2) S16 S33 S36/37 |
| NFPA 704 |
 4
3
2
|
| Flash point | 234,15 K (-39 °C) |
| Autoignition temperature |
458,15 K (185 °C) |
| Related compounds | |
| Related aldehydes | Formaldehyde Propionaldehyde |
| Related compounds | Ethylene oxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Acetaldehyde (systematically ethanal) is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part of their normal metabolism. It is also produced by oxidation of ethanol and is popularly believed to be the cause of hangovers.[2]
Contents |
Production
In 2003, global production was about 106 tons/year.[3] The main production method is the oxidation of ethylene via the Wacker process:
- 2 CH2=CH2 + O2 → 2 CH3CHO
Alternatively, hydration of acetylene, catalyzed by mercury salts gives ethenol, which tautomerizes to acetaldehyde. This industrial route was dominant prior to the Wacker process[4] It is also prepared at smaller levels by both the dehydrogenation and the oxidation of ethanol.
Reactions
Like many other carbonyl compounds, acetaldehyde tautomerizes to give the enol. The enol of acetaldehyde is vinyl alcohol (IUPAC name: ethenol):
- CH3CH=O
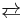 CH2=CHOH
CH2=CHOH
The equilibrium constant is only 6 x 10−5 at room temperature, so that the amount of the enol in a sample of acetaldehyde is very small.[5]

Condensation reactions
Because of its small size and its availability as the anhydrous monomer (unlike formaldehyde), it is a common electrophile in organic synthesis.[6] With respect to its condensation reactions, acetaldehyde is prochiral. It is mainly used as a source of the "CH3C+H(OH)" synthon in aldol and related condensation reactions.[7] Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives.[8] In one of the more spectacular condensation reactions, three equivalents of formaldehyde add to MeCHO to give pentaerythritol, C(CH2OH)4.[9]
In a Strecker reaction, acetaldehyde condenses with cyanide and ammonia to give, after hydrolysis, the amino acid alanine.[10] Acetaldehyde can condense with amines to yield imines, such as the condensation with cyclohexylamine to give N-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.[11]
It is also an important building block for the synthesis of heterocyclic compounds. A remarkable example is its conversion upon treatment with ammonia to 5-ethyl-2-methylpyridine ("aldehyde-collidine”).[12]
Acetal derivatives
Three molecules of acetaldehyde condense to form “paraldehyde,” a cyclic trimer containing C-O single bonds. The condensation of four molecules of acetaldehyde give the cyclic molecule called metaldehyde.
Acetaldehyde forms a stable acetal upon reaction with ethanol under conditions that favor dehydration. The product, CH3CH(OCH2CH3)2, is in fact called "acetal,"[13] although acetal is used more widely to describe other compounds with the formula RCH(OR')2.
Uses
Traditionally, acetaldehyde was mainly used as a precursor to acetic acid. This application has declined because acetic acid is made more efficiently from methanol by the Monsanto and Cativa processes. It is still heavily used. In terms of condensation reactions, acetaldehyde is an important precursor to pyridine derivatives, pentaerythritol, and crotonaldehyde. Urea and acetaldehyde combine to give a useful resin. Acetic anhydride reacts with acetaldehyde to give ethylidene diacetate, a precursor to vinyl acetate, which is used to produce polyvinyl acetate.
Biochemistry and health effects
In the liver, the enzyme alcohol dehydrogenase oxidizes ethanol into acetaldehyde, which is then further oxidized into harmless acetic acid by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of NAD+ to NADH[14]. In the brain, alcohol dehydrogenase has a minor role in the oxidation of ethanol to acetaldehyde. Instead, the enzyme catalase primarily oxidizes ethanol to acetaldehyde[14]. The last steps of alcoholic fermentation in bacteria, plants and yeast involve the conversion of pyruvate into acetaldehyde by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Tobacco addiction
Acetaldehyde is a significant constituent of tobacco smoke. It has been demonstrated to have a synergistic effect with nicotine, increasing the onset and tenacity of addiction to cigarette smoking, particularly in adolescents.[15][16]
Alzheimer's disease
People who have a genetic deficiency for the conversion of acetaldehyde into acetic acid may have a greater risk of Alzheimer's disease. "These results indicate that the ALDH2 deficiency is a risk factor for LOAD [late-onset Alzheimer's disease] …"[17]
Alcohol problems
Acetaldehyde derived from the consumption of ethanol binds to proteins to form adducts that are linked to organ disease.[18]
The drug disulfiram (Antabuse) prevents the oxidation of acetaldehyde to acetic acid, and it has the same unpleasant effect on drinkers. Antabuse is used as a deterrent for alcoholics who wish to stay sober.
Carcinogen
Acetaldehyde is a probable carcinogen in humans.[19] The International Agency for Research on Cancer states, "There is sufficient evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals."[20] In addition, acetaldehyde is damaging to DNA[21] and causes abnormal muscle development as it binds to proteins.[22]
A study of 818 heavy drinkers found that those who are exposed to more acetaldehyde than normal through a defect in the gene for alcohol dehydrogenase are at greater risk of developing cancers of the upper gastrointestinal tract and liver.[23]
Safety
Acetaldehyde is toxic when applied externally for prolonged periods, an irritant, and a probable carcinogen.[19] It is an air pollutant resulting from combustion, such as automotive exhaust and tobacco smoke. It is also created by thermal degradation of polymers in the plastics processing industry.[24]
See also
References
- ↑ SciFinderScholar (accessed Nov 4, 2009). Acetaldehyde (75-07-0) Substance Detail.
- ↑ How Hangovers Work, HowStuffWorks
- ↑ Marc Eckert, Gerald Fleischmann, Reinhard Jira, Hermann M. Bolt, Klaus Golka “Acetaldehyde” in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_031.pub2.
- ↑ Dmitry A. Ponomarev and Sergey M. Shevchenko (2007). "Hydration of Acetylene: A 125th Anniversary". J. Chem. Ed. 84 (10): 1725. doi:10.1021/ed084p1725. http://jchemed.chem.wisc.edu/HS/Journal/Issues/2007/OctACS/ACSSub/p1725.pdf.
- ↑ March, J. “Organic Chemistry: Reactions, Mechanisms, and Structures” J. Wiley, New York: 1992. ISBN 0-471-58148-8.
- ↑ Sowin, T. J.; Melcher, L. M. ”Acetaldehyde” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289
- ↑ Behrens, C.; Paquette, L. A. (2004), "N-Benzyl-2,3-Azetidinedione", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=V75P0106; Coll. Vol. 10: 41
- ↑ Walter, L. A. (1955), "1-(α-Pyridyl)-2-Propanol", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0757; Coll. Vol. 3: 757
- ↑ Schurink, H. B. J. (1941), "Pentaerythritol", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0425; Coll. Vol. 1: 425
- ↑ Kendall, E. C. McKenzie, B. F. (1941), "dl-Alanine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0021; Coll. Vol. 1: 21
- ↑ Wittig, G.; Hesse, A. (1988), "Directed Aldol Condensations: β-Phenylcinnamaldehyde", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0901; Coll. Vol. 6: 901
- ↑ Frank, R. L.; Pilgrim, F. J.; Riener, E. F. (1963), "5-Ethyl-2-Methylpyridine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV4P0451; Coll. Vol. 4: 451
- ↑ Adkins, H.; Nissen, B. H. (1941), "Acetal", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0001; Coll. Vol. 1: 1
- ↑ 14.0 14.1 Hipolito, L.; Sanchez, M. J.; Polache, A.; Granero, L. Brain metabolism of ethanol and alcoholism: An update. Curr. Drug Metab. 2007, 8, 716-727.
- ↑ Study Points to Acetaldehyde-Nicotine Combination in Adolescent Addiction
- ↑ Nicotine's addictive hold increases when combined with other tobacco smoke chemicals, UCI study finds
- ↑ "Mitochondrial ALDH2 Deficiency as an Oxidative Stress". Annals of the New York Academy of Sciences 1011: 36–44. April 2004. doi:10.1196/annals.1293.004. PMID 15126281. http://www3.interscience.wiley.com/journal/118765604/abstract?CRETRY=1&SRETRY=0. Retrieved 2009-08-13.
- ↑ Nakamura, K.; Iwahashi, K.; Furukawa, A.; Ameno, K.; Kinoshita, H.; Ijiri, I.; Sekine, Y.; Suzuki, K.; Iwata, Y.; Minabe, Y.; Mori, N. Acetaldehyde adducts in the brain of alcoholics. Arch. Toxicol. 2003, 77, 591.
- ↑ 19.0 19.1 Chemical Summary For Acetaldehyde, US Environmental Protection Agency
- ↑ International Agency for Rescarch on Cancer, World Health Organization. (1988). Alcohol drinking. Lyon: World Health Organization, International Agency for Research on Cancer. ISBN 92-832-1244-4. http://monographs.iarc.fr/ENG/Monographs/vol44/volume44.pdf. p3
- ↑ http://www.ist-world.org/ResultPublicationDetails.aspx?ResultPublicationId=2c488c559db74d8cae0c14ae5b65e14e
- ↑ Nicholas S. Aberle, II, Larry Burd, Bonnie H. Zhao and Jun Ren (2004). "Acetaldehyde-induced cardiac contractile dysfunction may be alleviated by vitamin B1 but not by vitamins B6 or B12". Alcohol & Alcoholism 39 (5): 450–454. doi:10.1093/alcalc/agh085. PMID 15304379. http://alcalc.oxfordjournals.org/cgi/content/full/39/5/450.
- ↑ Nils Homann, Felix Stickel, Inke R. König, Arne Jacobs, Klaus Junghanns, Monika Benesova, Detlef Schuppan, Susanne Himsel, Ina Zuber-Jerger, Claus Hellerbrand, Dieter Ludwig, Wolfgang H. Caselmann, Helmut K. Seitz Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers International Journal of Cancer Volume 118, Issue 8, Pages 1998-2002
- ↑ Smoking. (2006). Encyclopædia Britannica. Accessed 27 Oct 2006.
External links
- International Chemical Safety Card 0009
- National Pollutant Inventory - Acetaldehde
- NIOSH Pocket Guide to Chemical Hazards
- Methods for sampling and analysis
- IARC Monograph: "Acetaldehyde"
- Hal Kibbey, Genetic Influences on Alcohol Drinking and Alcoholism, Indiana University Research and Creative Activity, Vol. 17 no. 3.
- United States Food and Drug Administration (FDA) information for acetaldehyde