Paraquat
| Paraquat | |
|---|---|
 |
|
 |
|
|
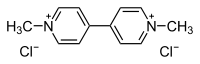
1,1'-dimethyl-4,4'-bipyridinium dichloride
|
|
|
Other names
paraquat dichloride; methyl viologen dichloride; Crisquat; Dexuron; Esgram; Gramuron; Ortho Paraquat CL; Para-col; Pillarxone; Tota-col; Toxer Total; PP148; Cyclone; Gramixel; Gramoxone; Pathclear; AH 501.
|
|
| Identifiers | |
| CAS number | 1910-42-5 |
|
SMILES
[Cl-].[Cl-].C[n+]1ccc(cc1)c2cc[n+](C)cc2
|
|
|
InChI
InChI=1/C12H14N2.2ClH/c1-
13-7-3-11(4-8-13)12- 5-9-14(2)10-6-12;;/ h3-10H,1-2H3;2*1H/ q+2;;/p-2/fC12H14N2.2Cl/ h;2*1h/qm;2*-1 |
|
| Properties | |
| Molecular formula | C12H14Cl2N2 |
| Molar mass | 257.16 g/mol |
| Appearance | off-white powder |
| Density | 1.25 g/cm3, solid |
| Melting point |
175 - 180 °C [1] |
| Boiling point |
> 300 °C [1] |
| Solubility in water | high |
| Hazards | |
| MSDS | Oxford MSDS |
| Main hazards | Toxic |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Paraquat is the trade name for N,N′-dimethyl-4,4′-bipyridinium dichloride, one of the most widely used herbicides in the world. Paraquat, a viologen, is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic to human beings when swallowed.
Contents |
Production
Pyridine is coupled with sodium in anhydrous ammonia to give 4,4'-bipyridine, which is then methylated with chloromethane to give the desired compound:[2]
History
Although first synthesized in 1882, paraquat's herbicidal properties were not recognized until 1955.[3] Paraquat was first produced for commercial purposes in 1961 by Sinon Corporation for ICI (now Syngenta), and is today among the most commonly used herbicides.
The European Union allowed paraquat in 2004. Sweden, supported by Denmark, Austria, and Finland, brought the European Union commission to court. On 11 July 2007, the court annulled the directive authorising paraquat as an active plant protection substance.[4]
Herbicide use
Paraquat is used as a quaternary ammonium herbicide, one of the most widely used herbicides in the world. It is quick-acting, non-selective, and kills green plant tissue on contact. It is redistributed within the plant, but does not harm mature bark. Being a herbicide, paraquat protects crops by controlling a wide range of annual and certain perennial weeds that reduce crop yield and quality by competing with the crop for water, nutrients, and light.
The key characteristics that distinguish the non-selective contact herbicide paraquat from other active ingredients used in plant protection products are:
- It is non-selective, which means it kills a wide range of annual grasses and broad-leaved weeds and the tops of established perennial weeds.
- It is very fast-acting.
- It is rain-fast within minutes of application.
- It becomes biologically inactive upon contact with soil.[5]
In the United States, paraquat is available primarily as a liquid in various strengths. It is classified as "restricted use," which means that it can be used only by licensed applicators. As with many chemicals, caution must be exercised during use. In the European Union, paraquat has been forbidden since July 11, 2007.
Mode of action as an herbicide
Paraquat inhibits photosynthesis. In light-exposed plants, it accepts electrons from photosystem I (more specifically Fd which is presented with electrons from PS I) and transfers them to molecular oxygen. In this manner, destructive reactive oxygen species are produced. In forming these reactive oxygen species, the oxidized form of paraquat is regenerated, and is again available to shunt electrons from photosystem I to start the cycle again.[6]
Scientific use
Paraquat is often used in science to catalyze the formation of reactive oxygen species (ROS). Paraquat will undergo redox cycling in vivo, being reduced by an electron donor such as NADPH, before being oxidized by an electron receptor such as dioxygen to produce superoxide, a major ROS.[7]
"Paraquat pot"
During the late 1970s, a controversial program sponsored by the US government sprayed paraquat on marijuana fields in Mexico.[8] Since much of this marijuana was subsequently smoked by Americans, the US government's "Paraquat Pot" program stirred much debate. Perhaps in an attempt to deter people from using marijuana, representatives of the program warned that spraying rendered the crop unsafe to smoke.
However, independent bodies have studied paraquat in this use. Jenny Pronczuk de Garbino,[9] stated: "no lung or other injury in marijuana users has ever been attributed to paraquat contamination". Also a United States Environmental Protection Agency manual states: "... toxic effects caused by this mechanism have been either very rare or nonexistent. Most paraquat that contaminates marijuana is pyrolyzed during smoking to dipyridyl, which is a product of combustion of the leaf material itself (including marijuana) and presents little toxic hazard."[10]
In suicide
A large majority (93%) of fatalities from paraquat poisoning are cases of intentional self-administration, i.e., suicides. In third world countries, paraquat is a "major suicide agent".[11] For instance, in Samoa from 1979-2001, 70% of suicides were by paraquat poisoning. In southern Trinidad from 1996-1997, 76% of suicides were by paraquat.[12]
The reason paraquat is such a widely used suicide agent in third-world countries is due to its widespread availability, low toxic dose (10 ml or 2 teaspoons is enough to kill) and relative low cost. There are campaigns to control or even ban paraquat outright, and there are moves to restrict its availability by requiring user education and the locking up of paraquat stores.
Toxicity
Pure paraquat, when ingested, is highly toxic to mammals, including humans; potentially leading to acute respiratory distress syndrome (ARDS), and there are no specific antidotes. However, fuller's earth or activated charcoal is an effective treatment, if taken in time. Death may occur up to 30 days after ingestion. Diluted paraquat used for spraying is less toxic; thus, the greatest risk of accidental poisoning is during mixing and loading paraquat for use.[3]
In acute toxicity studies using laboratory animals, paraquat has been shown to be highly toxic by the inhalation route and has been placed in Toxicity Category I (the highest of four levels) for acute inhalation effects. However, the EPA has determined that particles used in agricultural practices (400 to 800 μm) are well beyond the respirable range and therefore inhalation toxicity is not a toxicological endpoint of concern. Paraquat is toxic (Category II) by the oral route and moderately toxic (Category III) by the dermal route. Paraquat will cause moderate to severe eye irritation and minimal dermal irritation, and has been placed in Toxicity Categories II and IV (slightly toxic) respectively for these effects.[13]
Even a single swig, immediately spat out, can cause death from fibrous tissue developing in the lungs, leading to asphyxiation.[14]
According to the Center for Disease Control, ingesting paraquat causes symptoms such as liver, lung, heart, and kidney failure within several days to several weeks that can lead to death up to 30 days after ingestion. Those who suffer large exposures are unlikely to survive. Chronic exposure can lead to lung damage, kidney failure, heart failure, and oesophageal strictures.[15] Accidental deaths and suicides from paraquat ingestion are relatively common. For example, there have been 18 deaths in Australia from paraquat poisoning since 2000.[16]
Paraquat-induced toxicity in rats has also been linked to Parkinson's-like neurological degenerative mechanisms.[17] A study by the Buck Institute showed a connection between exposure to paraquat and iron in infancy and mid-life Parkinson's in laboratory mice.[18]
Long term exposures to paraquat would most likely cause lung and eye damage, but reproductive/fertility damage was not found by the EPA in their review. Some suspect a possible link to a greater incidence of Parkinson's disease.
References
- ↑ 1.0 1.1 "Paraquat dichloride". International Programme on Chemical Safety. October 2001. http://www.inchem.org/documents/icsc/icsc/eics0005.htm.
- ↑ "Paraquat and Diquat". IPCS INCHEM. http://www.inchem.org/documents/ehc/ehc/ehc39.htm.
- ↑ 3.0 3.1 "Paraquat". Pesticides News 32: 20-21. 1996.
- ↑ COURT OF FIRST INSTANCE OF THE EUROPEAN COMMUNITIES, PRESS RELEASE No° 45/07
- ↑ Revkin, A. C. 1983. Paraquat: A potent weed killer is killing people. Science Digest 91(6):36-38, 42, 100-104.
- ↑ Summers L.A. (1980) The Bipyridinium Herbicides. Academic Press, New York, NY.
- ↑ Bus et al. Paraquat: model for oxidant-initiated toxicity. Environmental Health Perspectives (1984) vol. 55 pp. 37-46
- ↑ Panic over Paraquat, Time Magazine, Monday, May 1, 1978
- ↑ Pronczuk de Garbino J, Epidemiology of paraquat poisoning, in: Bismuth C, and Hall AH (eds), Paraquat Poisoning: Mechanisms, Prevention, Treatment, pp. 37-51, New York: Marcel Dekker, 1995.
- ↑ Reigart, J. Routt and Roberts, James R. Recognition and Management of Pesticide Poisonings, 5th edition. Washington, DC: United States Environmental Protection Agency, 1999. Book available online
- ↑ "Active Ingredient fact sheet, Paraquat". Pesticide News 32: 20-1. 1996.
- ↑ http://www.pan-germany.org/download/fact_paraquat2.pdf
- ↑ United States Environmental Protection Agency, [1], accessed 16 August 2007.
- ↑ Buzik, Shirley C.; Schiefer, H. Bruno; Irvine, Donald G. (1997). Understanding Toxicology: Chemicals, Their Benefits and Risks. Boca Raton: CRC Press. p. 31. ISBN 0-8493-2686-9.
- ↑ Center for Disease Control, Facts about Paraquat, accessed 13 October 2006.
- ↑ "Poisoned Latrobe," Gary Stevens, Valley Express Feb. 8, 2008.
- ↑ K. Ossowska, M. S'Mialowska, K. Kuter, J. Wieron'ska, B. Zieba, J. Wardas, P. Nowak, J. Dabrowska, A. Bortel, I. Biedka, G. Schulze and H. Rommelspacher (2006). "Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: Implications for Parkinson's disease". Neuroscience 141 (4): 2155–2165. doi:10.1016/j.neuroscience.2006.05.039. PMID 16797138.
- ↑ Buck Institute for Aging Research (June 2007). "Combined Exposure to Environmental Toxics Accelerates Age-related Development of Parkinson's Disease in Mice". Press release. http://www.buckinstitute.org/site/index.php?option=com_content&task=view&id=428.
Further reading
- Slade, P. (1966). "The Fate of Paraquat Applied to Plants". Weed Research 6: 158. doi:10.1111/j.1365-3180.1966.tb00876.x.
- Smith, S. N.; Lyon, A. J. E.; Sahid, Ismail BIN (1976). "The Breakdown of Paraquat and Diquat by Soil Fungi". New Phytologist 77: 735. doi:10.1111/j.1469-8137.1976.tb04668.x.
External links
- "Paradox Product". Sinon do Brasil. http://www.sinon.com.br/english/products_paradox.php.
- "Stop Paraquat". The Berne Declaration. http://www.evb.ch/en/f25000087.html.
- "The Paraquat Information Center". Syngenta Crop Protection AG. http://www.paraquat.com.
|
||||||||||||||||||||||||||||||||