Ricin

Ricin (pronounced /ˈraɪsɨn/) is a protein that is extracted from the castor bean (Ricinus communis). Ricin may cause allergic reactions, and is toxic, though the severity depends on the route of exposure.
The LD50 of ricin is around 22 micrograms per kilogram (1.76mg for an average adult, around 1/228 of a standard aspirin tablet (0.4g gross)) in humans if exposure is from injection or inhalation.[1] Oral exposure to ricin is far less toxic and lethal dose can be up to 20-30mg/kg.
Contents |
Toxicity
Ricin is poisonous if inhaled, injected, or ingested, acting as a toxin by the inhibition of protein synthesis. It is resistant, but not impervious, to digestion by peptidases. By ingestion, the pathology of ricin is largely restricted to the gastrointestinal tract where it may cause mucosal injuries; with appropriate treatment, most patients will make a full recovery.[2] Because the symptoms are caused by failure to make protein, they emerge only after a variable delay from a few hours to a full day after exposure. An antidote[3] and a vaccine[4] have been manufactured by military organizations. Symptomatic and supportive treatment is available. Long term organ damage is likely in survivors. Ricin causes severe diarrhea and victims can die of shock. Abrin is a similar toxin, found in the highly ornamental rosary pea.
Deaths caused by ingestion of castor plant seeds are rare, partly because of the indigestible capsule, and partly because ricin can be digested (although it is resistant).[5] The pulp from eight beans is considered toxic for an adult.[6] A solution of saline and glucose has been used to treat ricin overdose.[7] The case experience is not as negative as popular perception would indicate.[8]
Overdosage
Most acute poisoning episodes in humans are the result of oral ingestion of castor beans, 5-20 of which could prove fatal to an adult. Victims often manifest nausea, emesis, diarrhea, tachycardia, hypotension and seizures persisting for up to a week. Blood, plasma or urine ricin concentrations may be measured to confirm diagnosis.[9]
Biochemistry
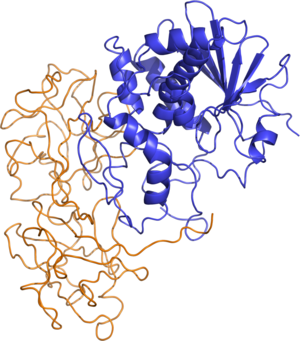
Ricin is classified as a type 2 ribosome inactivating protein (RIP). Whereas Type 1 RIPs consist of a single enzymatic protein chain, Type 2 RIPs, also known as holotoxins, are heterodimeric glycoproteins. Type 2 RIPs consist of an A chain that is functionally equivalent to a Type 1 RIP, covalently connected by a single disulfide bond to a B chain that is catalytically inactive, but serves to mediate entry of the A-B protein complex into the cytosol. Both Type 1 and Type 2 RIPs are functionally active against ribosomes in vitro, however only Type 2 RIPs display cytoxicity due to the lectin properties of the B chain. In order to display its ribosome inactivating function, the ricin disulfide bond must be reductively cleaved.[10]
Structure
The tertiary structure of ricin was shown to be a globular, glycosylated heterodimer of approximately 60-65 kDA.[5] Ricin toxin A chain (RTA) and ricin toxin B chain (RTB) are of similar molecular weight, approximately 32 kDA and 34 kDA respectively.
- Ricin A Chain is an N-glycoside hydrolase composed of 267 amino acids.[11] It has three structural domains with approximately 50% of the polypeptide arranged into alpha-helices and beta-sheets.[12] The three domains form a pronounced cleft that is the active site of RTA.
- Ricin B Chain is a lectin composed of 262 amino acids that is able to bind terminal galactose residues on cell surfaces.[13] RTB form a bilobal, barbell-like structure lacking alpha-helices or beta-sheets where individual lobes contain three subdomains. At least one of these three subdomains in each homologous lobe possesses a sugar-binding pocket that gives RTB its functional character.
Many plants such as barley have the A chain but not the B chain. People do not get sick from eating large amounts of such products, as ricin A is of extremely low toxicity as long as the B chain is not present.
Entry into the cytosol
The ability of ricin to enter the cytosol depends on hydrogen bonding interactions between RTB amino acid residues and complex carbohydrates on the surface of eukaryotic cells containing either terminal N-acetyl galactosamine or beta-1,4-linked galactose residues. Additionally, the mannose-type glycans of ricin are able to bind cells that express mannose receptors.[14] Experimentally, RTB has been shown to bind to the cell surface on the order of 106-108 ricin molecules per cell surface.[15]
The profuse binding of ricin to surface membranes allows internalization with all types of membrane invaginations. Experimental evidence points to ricin uptake in both clathrin-coated pits, as well as clathrin-independent pathways including caveolae and macropinocytosis.[16][17] Vesicles shuttle ricin to endosomes that are delivered to the Golgi apparatus. The active acidification of endosomes are thought to have little effect on the functional properties of ricin. Because ricin is stable over a wide pH range, degradation in endosomes or lysosomes offer little or no protection against ricin.[18] Ricin molecules are thought to follow retrograde transport through the Golgi and enter the endoplasmic reticulum (ER).
For ricin to function cytotoxically, RTA must be reductively cleaved from RTB in order to release a steric block of the RTA active site. Currently, it is unknown whether this takes place in the ER or in the cytosol. It is speculated that within the ER, RTA utilizes the endoplasmic reticulum-associated protein degradation (ERAD) pathway that exists to eject misfolded proteins to the cytosol.[19] Chaperones participating in ERAD may recognize RTA as misfolded native protein and translocate it into the cytosol. Additionally, RTA resists degradation by ubiquitination that often occurs with misfolded proteins by maintaining a low content of lysine residues, the usual attachment sites for ubiquitin.[20] In the cytosol, RTA is free to exert its toxicity on ribosomes.
Ribosome inactivation
Study of the N-glycosidase activity of ricin was pioneered by Endo and Tsurugi[21] who showed that RTA cleaves a glycosidic bond within the large rRNA of the 60S subunit of eukaryotic ribosomes. They subsequently showed RTA specifically and irreversibly hydrolyses the N-glycosidic bond of the adenine residue at position 4324 (A4324) within the 28S rRNA, but leaves the phosphodiester backbone of the RNA intact.[22] The ricin targets A4324 that is contained in a highly conserved sequence of 12 nucleotides universally found in eukaryotic ribosomes. The sequence, 5’-AGUACGAGAGGA-3’, termed the sarcin-ricin loop, is important in binding elongation factors during protein synthesis.[23] The depurination event rapidly and completely inactivates the ribosome, resulting in toxicity from inhibited protein synthesis. A single RTA molecule in the cytosol is capable of depurinating approximately 1500 ribosomes per minute.
Depurination reaction
Within the active site of RTA, there exist several invariant amino acid residues involved in the depurination of ribosomal RNA.[18] Although the exact mechanism of the event is unknown, key amino acid residues identified include tyrosine at positions 80 and 123, glutamic acid at position 177, and arginine at position 180. In particular, Arg180 and Glu177 have been shown to be involved in the catalytic mechanism, and not substrate binding, with enzyme kinetic studies involving RTA mutants. The model proposed by Mozingo and Robertus,[24] based x-ray structures, is as follows:
- Sarcin-ricin loop substrate binds RTA active site with target adenine stacking against tyr80 and tyr123.
- Arg180 is positioned such that it can protonate N-3 of adenine and break the bond between N-9 of the adenine ring and C-1’ of the ribose.
- Bond cleavage results in an oxycarbonium ion on the ribose, stabilized by Glu177.
- N-3 protonation of adenine by Arg180 allows deprotonation of a nearby water molecule.
- Resulting hydroxyl attacks ribose carbonium ion.
- Depurination of adenine results in a neutral ribose on an intact phosphodiester RNA backbone.
Manufacture
Ricin is easily purified from castor-oil manufacturing waste. The aqueous phase left over from the oil extraction process is called waste mash. It contains about 5-10% ricin by weight. Separation requires only simple chromatographic techniques.
Patented extraction process
A process for extracting ricin has been described in a patent.[25] The described extraction method is very similar to that used for the preparation of soy protein isolates.
The patent was removed from the United States Patent and Trademark Office (USPTO) database sometime in 2004, but it is still available online through international patent databases.[26][27] Modern theories of protein chemistry cast doubt on the effectiveness of the methods disclosed in the patent.[28]
Potential medicinal use
Some researchers have speculated about using ricins in the treatment of cancer, as a so-called "magic bullet" to destroy targeted cells:[29] Because ricin is a protein, it can be genetically linked to a monoclonal antibody to target malignant cells recognized by the antibody. The major problem with ricin is that its native internalization sequences are distributed throughout the protein. If any of these native internalization sequences are present in a therapeutic, then the drug will be internalized by, and kill, untargeted epithelial cells as well as targeted cancer cells.
Some researchers hope that modifying ricin will sufficiently lessen the likelihood that the ricin component of these immunotoxins will cause the wrong cells to internalize it, while still retaining its cell-killing activity when it is internalized by the targeted cells. Generally, however, ricin has been superseded for medical purposes by more practical fragments of bacterial toxins, such as diphtheria toxin, which is used in denileukin diftitox, an FDA-approved treatment for leukemia and lymphoma. No approved therapeutics contain ricin.
A promising approach is also to use the non-toxic B subunit as a vehicle for delivering antigens into cells thus greatly increasing their immunogenicity. Use of ricin as an adjuvant has potential implications for developing mucosal vaccines.
Incidents involving ricin
Ricin has been involved in a number of incidents, including the high-profile assassination of Georgi Markov using a weapon disguised as an umbrella.
Use as a chemical/biological warfare agent
The United States investigated ricin for its military potential during the First World War. At that time it was being considered for use either as a toxic dust or as a coating for bullets and shrapnel. The dust cloud concept could not be adequately developed, and the coated bullet/shrapnel concept would violate the Hague Convention of 1899 (adopted in U.S. law at 32 Stat. 1803), specifically Annex § 2, Ch.1, Article 23, stating "...it is especially prohibited...[t]o employ poison or poisoned arms;".[30] The First World War ended before the U.S. weaponized ricin.
During the Second World War the United States and Canada undertook studying ricin in cluster bombs. Though there were plans for mass production and several field trials with different bomblet concepts, the end conclusion was that it was no more economical than using phosgene. This conclusion was based on comparison of the final weapons rather than ricin's toxicity (LCt50 ~40 mg·min/m3). Ricin was given the military symbol W or later WA. Interest in it continued for a short period after the Second World War, but soon subsided when the U.S. Army Chemical Corps began a program to weaponize sarin.
The Soviet Union also possessed weaponized ricin. There were speculations that the KGB even used it outside of the Soviet bloc; however, this was never proven. In 1978, the Bulgarian dissident Georgi Markov was assassinated by Bulgarian secret police who surreptitiously 'shot' him on a London street with a modified umbrella using compressed gas to fire a tiny pellet contaminated with ricin into his leg.[31] He died in a hospital a few days later; his body was passed to a special poison branch of the British Ministry of Defence (MOD) that discovered the pellet during an autopsy. The prime suspects were the Bulgarian secret police: Georgi Markov had defected from Bulgaria some years previously and had subsequently written books and made radio broadcasts which were highly critical of the Bulgarian communist regime. However, it was believed at the time that Bulgaria would not have been able to produce the pellet, and it was also believed that the KGB had supplied it. The KGB denied any involvement although high-profile KGB defectors Oleg Kalugin and Oleg Gordievsky have since confirmed the KGB's involvement. Earlier, Soviet dissident Aleksandr Solzhenitsyn also suffered (but survived) ricin-like symptoms after a 1971 encounter with KGB agents.[32] See also: Alexander Litvinenko.
Despite ricin's extreme toxicity and utility as an agent of chemical/biological warfare, it is extremely difficult to limit the production of the toxin. Under both the 1972 Biological Weapons Convention and the 1997 Chemical Weapons Convention, ricin is listed as a schedule 1 controlled substance. Despite this, more than 1 million tonnes of castor beans are processed each year, and approximately 5% of the total is rendered into a waste containing high concentrations of ricin toxin.[33]
To put ricin used as a weapon into perspective, it is worth noting that as a biological weapon or chemical weapon, ricin may not be considered very powerful in comparison with other agents such as botulinum or anthrax. Furthermore, the quantity of ricin required to achieve LD50 over a large geographic area is significantly more than an agent such as anthrax (tonnes of ricin vs. only kilogram quantities of anthrax).[34] Hence, a military willing to use biological weapons and having advanced resources would rather use either of the latter instead. Ricin is easy to produce, but is not as practical nor likely to cause as many casualties as other agents.[2] Ricin is inactivated (the protein changes structure and becomes less dangerous) much more readily than anthrax spores, which may remain lethal for decades. (Jan van Aken, an expert on biological weapons explained in an interview with the German magazine Der Spiegel that he judges it rather reassuring that Al Qaeda experimented with ricin as it suggests their inability to produce botulin or anthrax.)
The major reason it is dangerous is that it is very easy to obtain (the castor bean plant is a common ornamental, and can be grown at home without any special care). Ricin is actually several orders of magnitude less toxic than botulinum or tetanus toxin, but those are more difficult to obtain.
References
- ↑ "EFSA Scientific Opinion: Ricin (from Ricinus communis) as undesirable substances in animal feed [1] - Scientific Opinion of the Panel on Contaminants in the Food Chain". Efsa.europa.eu. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902083375.htm. Retrieved 2010-09-01.
- ↑ 2.0 2.1 Schep LJ, Temple WA, Butt GA, Beasley MD (2009). "Ricin as a weapon of mass terror--separating fact from fiction". Environ Int 35 (8): 1267–71. doi:10.1016/j.envint.2009.08.004. PMID 19767104.
- ↑ Rincon, Paul (2009-11-11). "http://news.bbc.co.uk/1/hi/sci/tech/8351666.stm". BBC News. http://news.bbc.co.uk/1/hi/sci/tech/8351666.stm. Retrieved 2010-09-01.
- ↑ Karen Fleming-Michael (2005-09-01). "http://www.dcmilitary.com/dcmilitary_archives/stories/090105/36813-1.shtml". Dcmilitary.com. http://www.dcmilitary.com/dcmilitary_archives/stories/090105/36813-1.shtml. Retrieved 2010-09-01.
- ↑ Aplin PJ, Eliseo T (1997). "Ingestion of castor oil plant seeds". Med. J. Aust. 167 (5): 260–1. PMID 9315014.
- ↑ Wedin GP, Neal JS, Everson GW, Krenzelok EP (1986). "Castor bean poisoning". The American journal of emergency medicine 4 (3): 259–61. doi:10.1016/0735-6757(86)90080-X. PMID 3964368.
- ↑ Kopferschmitt J, Flesch F, Lugnier A, Sauder P, Jaeger A, Mantz JM (1983). "Acute voluntary intoxication by ricin". Human toxicology 2 (2): 239–42. doi:10.1177/096032718300200211. PMID 6862467.
- ↑ Rauber A, Heard J (1985). "Castor bean toxicity re-examined: a new perspective". Veterinary and human toxicology 27 (6): 498–502. PMID 4082461.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1381-1383.
- ↑ Wright HT, Robertus JD (1987). "The intersubunit disulfide bridge of ricin is essential for cytotoxicity". Arch Biochem Biophys 256 (1): 280–4. doi:10.1016/0003-9861(87)90447-4. PMID 3606124.
- ↑ Olnes S, Pihl A (1973). "Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis". Biochemistry 12: 3121–26. doi:10.1021/bi00740a028.
- ↑ Weston SA, Tucker AD, Thatcher DR, et al. (1994). "X-ray structure of recombinant ricin A-chain at 1.8 A resolution". J Mol Biol 244 (4): 410–22. doi:10.1006/jmbi.1994.1739. PMID 7990130.
- ↑ Wales R, Richardson PT, Robers LM, Woodland HR, et al. (1991). "Mutational analysis of the galactose binding ability of recombinant ricin b chain". J Biol Chem 266: 19172–79.
- ↑ Magnusson AS, Kjeken R, Berg T (1993). "Characterization of two distinct pathways of endocytosis of ricin by rat liver endothelial cells". Exp Cell Res 205 (1): 118–25. doi:10.1006/excr.1993.1065. PMID 8453986.
- ↑ Sphyris N, Lord JM, Wales R, et al. (1995). "Mutational analysis of the ricinus lectin b-chains: Galactose-binding ability of the gamma subdomain of ricinus communis agglutin b-chain". J Biol Chem 270 (35): 20292–97. doi:10.1074/jbc.270.35.20292. PMID 7657599.
- ↑ Moya M, Dautry-Varsat A, Goud B, et al. (1985). "Inhibition of coated pit formatin in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin". J Cell Biol 101 (2): 548–59. doi:10.1083/jcb.101.2.548. PMID 2862151.
- ↑ Nichols, BJ, Lippincott-Schwartz J (2001). "Endocytosis without clathrin coats". Trends Cell Biol 11 (10): 406–12. doi:10.1016/S0962-8924(01)02107-9. PMID 11567873.
- ↑ 18.0 18.1 Lord MJ, Jolliffe NA, Marsden CJ, et al. (2003). "Ricin Mechanisms of Cytotoxicity". Toxicol Rev 22 (1): 53–64. doi:10.2165/00139709-200322010-00006. PMID 14579547.
- ↑ Roberts LM, Smith DC (2004). "Ricin: the endoplasmic reticulum connection". Toxicon 44 (5): 469–72. doi:10.1016/j.toxicon.2004.07.002. PMID 15450920.
- ↑ Deeks ED, Cook JP, Day PJ, et al. (2002). "The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol". Biochemistry 41 (10): 3405–13. doi:10.1021/bi011580v. PMID 11876649.
- ↑ Endo Y, Tsurugi K (1987). "RNA N-glycosidase activity of ricin A-chain: mechanism of action of the toxic lectin ricin on eukaryotic ribosomes". J Biol Chem 262 (17): 8128–30. PMID 3036799.
- ↑ Endo Y, Tsurugi K (1998). "The RNA N-glycosidase activity of ricin A chain". J Biol Chem 263 (18): 8735–9. PMID 3288622.
- ↑ Sperti S, Montanaro L, Mattioli A, et al. (1973). "Inhibition by ricin of protein synthesis in vitro: 60 S ribosomal subunit as the target of the toxin.". Biochem J 136 (3): 813–5. PMID 4360718.
- ↑ Monzingo AF, Robertus JD (1992). "X-ray analysis of substrate analogs in the ricin A-chain active site". J Mol Biol 244 (4): 410–22. doi:10.1006/jmbi.1994.1739. PMID 7990130.
- ↑ "Preparation of Toxic Ricin", U.S. Patent 3,060,165, assigned to the U.S. Secretary of the Army, inventors: Harry L. Craig, O.H. Alderks, Alsoph H. Corwin, Sally H. Dieke, and Charlotte Karel (granted October 23, 1962)
- ↑ "Harry L. Craig, O.H. Alderks, Alsoph H. Corwin, Sally H. Dieke, and Charlotte Karel, US Patent 3,060,165, "Preparation of Toxic Ricin", granted October 23, 1962". V3.espacenet.com. http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=US3060165&QPN=US3060165. Retrieved 2010-09-01.
- ↑ "Ricin Patent". Cryptome.org. 2004-03-12. http://cryptome.org/ricin-patent.htm. Retrieved 2010-09-01.
- ↑ John Pike. "http://www.globalsecurity.org/org/nsn/nsn-040723.htm". Globalsecurity.org. http://www.globalsecurity.org/org/nsn/nsn-040723.htm. Retrieved 2010-09-01.
- ↑ Lord MJ, Jolliffe NA, Marsden CJ, et al. (2003). "Ricin. Mechanisms of cytotoxicity". Toxicological reviews 22 (1): 53–64. PMID 14579547.
- ↑ "The Avalon Project — Laws of War : Laws and Customs of War on Land (Hague II); July 29, 1899". Avalon.law.yale.edu. http://avalon.law.yale.edu/19th_century/hague02.asp#art23. Retrieved 2010-09-01.
- ↑ "Ricin and the umbrella murder". CNN. January 7, 2003. http://www.cnn.com/2003/WORLD/europe/01/07/terror.poison.bulgarian/. Retrieved 2008-03-15.
- ↑ D.M. Thomas, Alexander Solzhenitsyn: A Century in His Life, 368-378
- ↑ "http://www.ansci.cornell.edu/plants/toxicagents/ricin.html". Ansci.cornell.edu. http://www.ansci.cornell.edu/plants/toxicagents/ricin.html. Retrieved 2010-09-01.
- ↑ Kortepeter, M.G. & Parker, G.W. (1999). Potential biological weapons threats. U.S. Army Medical Research Institute of Infectious Diseases, 5(4), 523-527
External links
- Castor bean information at Purdue University
- ricin information at Department of Health (United Kingdom)
- ricin information at Cornell University
- Medical research on ricin at BBC
- Chemical Review at United States Army
- Ricin - Emergency Preparations at CDC
- BioHealthBase Bioinformatics Resource Center Ricin bioinformatics resource
|
|||||||||||||
|
|||||||||||||||||||||||||||||||||