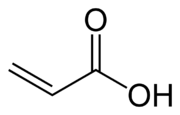
Acrylic acid
| Acrylic acid[1] | |
|---|---|
 |
|
 |
|
|
propenoic acid
|
|
|
Other names
acroleic acid
ethylenecarboxylic acid propene acid propenoic acid vinylformic acid |
|
| Identifiers | |
| CAS number | 79-10-7 |
| PubChem | 6581 |
| RTECS number | AS4375000 |
|
SMILES
C=CC(=O)O
|
|
| Properties | |
| Molecular formula | C3H4O2 |
| Molar mass | 72.06 g mol−1 |
| Appearance | clear, colorless liquid |
| Density | 1.051 g/mL |
| Melting point |
14 °C, 287 K, 57 °F |
| Boiling point |
141 °C, 414 K, 286 °F |
| Solubility in water | Miscible |
| Acidity (pKa) | 4.25 |
| Viscosity | 1.3 cP at 20 °C (68 °F) |
| Hazards | |
| MSDS | MSDS |
| R-phrases | R10 R20/21/22 R35 R50 |
| S-phrases | S26 S36/37/39 S45 S61 |
| Flash point | 68 °C (154 °F) |
| Related compounds | |
| Other anions | acrylate |
| Related carboxylic acids | acetic acid propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid crotonic acid |
| Related compounds | propenol propionaldehyde acrolein methyl acrylate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than one billion kilograms are produced annually.[2]
Contents |
Production
Acrylic acid is produced from propene (aka propylene) which is a byproduct of ethylene and gasoline production.
- CH2=CHCH3 + 1.5 O2 → CH2=CHCO2H + H2O
Because acrylic acid and its esters have long been valued commercially, many other methods have been developed but most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene ("Reppe chemistry"):
- HCCH + CO + H2O → CH2=CHCO2H
This method requires nickel carbonyl and high pressures of carbon monoxide. It was once manufactured by the hydrolysis of acylonitrile which is derived from propene by ammoxidation, was abandoned because the method cogenerates ammonium derivatives. Other now abandoned precursors to acrylic acid include ethenone and ethylene cyanohydrin.[2]
Reactions and uses
Acrylic acid undergoes the typical reactions of a carboxylic acid and, when reacted with an alcohol, it will form the corresponding ester. The esters and salts of acrylic acid are collectively known as acrylates (or propenoates). The most common alkyl esters of acrylic acid are methyl-, butyl-, ethyl-, and 2-ethylhexyl-acrylate.
Acrylic acid and its esters readily combine with themselves or other monomers (e.g. amides, acrylonitrile, vinyl, styrene, and butadiene) by reacting at their double bond, forming homopolymers or copolymers which are used in the manufacture of various plastics, coatings, adhesives, elastomers, as well as floor polishes, and paints.
Safety
Acrylic acid is severely irritating and corrosive to the skin and the respiratory tract. Eye contact can result in severe and irreversible injury. Low exposure will cause minimal or no health effects, while high exposure could result in pulmonary edema. LD50 = 340 mg/kg (rat, oral).
See also
- Methacrylic acid
- Acryloyl chloride
- Acrylamide
- Acrylate polymers
- Sodium polyacrylate
- Acryloyl group
References
- ↑ Merck Index, 11th Edition, 124.
- ↑ 2.0 2.1 Takashi Ohara, Takahisa Sato, Noboru Shimizu, Günter Prescher Helmut Schwind, Otto Weiberg, Klaus Marten, Helmut Greim “Acrylic Acid and Derivatives” in Ullmann's Encyclopedia of Industrial Chemistry 2003, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a01_161.pub2