Dipolar bond
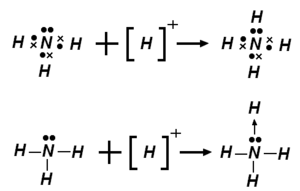
A dipolar bond[1], also known as coordinate link[2], coordinate covalent bond[3], dative bond[4], or semipolar bond, is a description of covalent bonding between two atoms in which both electrons shared in the bond come from the same atom. The distinction from ordinary covalent bonding is artificial, but the terminology is popular in textbooks, especially those describing coordination compounds. Once such a bond has been formed, its strength and description is no different from that of other polar covalent bonds.
Dipolar bonds occur when a Lewis base (an electron donor or giver) donates a pair of electrons to a Lewis acid (an electron acceptor) to give a so-called adduct. The process of forming a dipolar bond is called coordination. The electron donor acquires a positive formal charge, while the electron acceptor acquires a negative formal charge.
Contents |
Examples
Classically, any compound that contains a lone pair of electrons is capable of forming a dipolar bond. The bonding in diverse chemical compounds can be described as coordinate covalent bonding.
- Carbon monoxide (CO) can be viewed as containing one coordinate bond and two "normal" covalent bonds between the carbon atom and the oxygen atom. This highly unusual description illustrates the flexibility of this bonding description. Thus in CO, carbon is the electron acceptor and oxygen is the electron donor.
- Beryllium dichloride (BeCl2) is described as electron deficient in the sense that the triatomic species (which does exist in the gas phase) features Be centers with four valence electrons. When treated with excess chloride, the Be2+ ion binds four chloride ions to form tetrachloroberyllate anion, BeCl42-, wherein all ions achieve the octet configuration of electrons.
Coordination compounds
Dipolar bonding is popularly used to describe coordination complexes, especially involving metal ions. In such complexes, several Lewis bases "donate" their "free" pairs of electrons to an otherwise naked metal cation, which acts as a Lewis acid and "accepts" the electrons. Dipolar bonds form and the resulting compound is called a coordination complex, and the electron donors are called ligands. A more useful description of bonding in coordination compounds is provided by Ligand Field Theory, which embraces molecular orbitals as a description of bonding in such polyatomic compounds.
Many chemical compounds can serve as ligands. Often these contain oxygen, sulfur, nitrogen, and halide ions. The most common ligand is water (H2O), which forms coordination complexes with metal ions (like the hexaaquacopper(II) ion, [Cu(H2O)6]2+). Ammonia (NH3) is a common ligand. So are some anions, especially fluoride (F−), chloride (Cl−), and cyanide (CN−).
Important Points
- The atom which donates the pair of electrons is termed as Donor
- The atom which accepts the pair of electrons is termed as Acceptor
- The pair of electrons shared in this type of bond is the lone pair of the Donor
- The Donor develops a positive charge and the Acceptor develops a negative charge
- Although coordinate compounds do not ionize in water their physical properties are intermediate
between those of ionic and covalent compounds
- The coordinate bond is rigid and directional, like covalent bond
- Coordinate compounds exhibit stereoisomerism
See also
- Chemical bond
- Sigma bond
- Pi bond
- Delta bond
References
- ↑ IUPAC Gold Book internet edition: "dipolar bond".
- ↑ IUPAC Gold Book internet edition: "coordinate link".
- ↑ IUPAC Gold Book internet edition: "coordinate covalent bond".
- ↑ IUPAC Gold Book internet edition: "dative bond".
External links
|
||||||||||||||||||||||||