Potassium cyanide
| Potassium cyanide | |
|---|---|
 |
|
 |
|
|
Potassium cyanide
|
|
| Identifiers | |
| CAS number | 151-50-8 |
| EC number | 205-792-3 |
| UN number | 1680 |
| RTECS number | TS8750000 |
| Properties | |
| Molecular formula | KCN |
| Molar mass | 65.12 g/mol |
| Appearance | White crystalline solid deliquescent |
| Density | 1.52 g/cm3 |
| Melting point |
634.5 °C |
| Boiling point |
1625 °C |
| Solubility in water | 71.6 g/100 ml (25 °C) 100 g/100 mL (100 °C) |
| Solubility in methanol | 4.9 g/100 mL (20 °C) |
| Solubility in glycerol | soluble |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−131.5 kJ/mol |
| Standard molar entropy S |
127.8 J K−1 mol−1 |
| Hazards | |
| MSDS | ICSC 0671 |
| EU Index | 006-007-00-5 |
| EU classification | Very toxic (T+) Dangerous for the environment (N) |
| R-phrases | R26/27/28, R32, R50/53 |
| S-phrases | (S1/2), S7, S28, S29, S45, S60, S61 |
| NFPA 704 |
 0
4
0
|
| Flash point | Non-flammable |
| LD50 | 5–10 mg/kg (oral in rats, mice, rabbits)[1] |
| Related compounds | |
| Other anions | Potassium cyanate Potassium thiocyanate |
| Other cations | Sodium cyanide |
| Related compounds | Hydrogen cyanide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and buffing.[2]
KCN is highly toxic. The moist solid emits small amounts of hydrogen cyanide due to hydrolysis, which smells like bitter almonds. Not everyone, however, can smell this odor: the ability to do so is a genetic trait.[3] It is used by entomologists as a killing agent in collecting jars, as most insects succumb within seconds, minimizing damage of even highly fragile specimens.
Contents |
Production
KCN is produced by treating hydrogen cyanide with potassium hydroxide,
- HCN + KOH → KCN + H2O
or by treating formamide with potassium hydroxide:
- HCONH2 + KOH → KCN + 2H2O
Approximately 50,000 tons are produced yearly.[2]
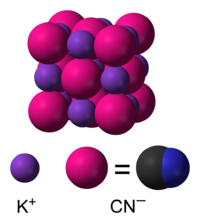
Structure
In aqueous solution, KCN is dissociated into hydrated K+ ions and CN−. As a solid, the salt crystallizes such that the cations and anions organize like Na+ and Cl− in NaCl. The cations and anions six-coordinate. Each K+ is linked to two pi-bonds of the CN− as well as two links each to C and N each. Since CN− is diatomic, the symmetry of the solid is lower than that in NaCl. The cyanide anions form sheets. The CN− ions rapidly rotate in the solid at ambient temperature such that the time averaged shape of the CN− ions is spherical.[4]
Applications
In gold mining, KCN and NaCN form water-soluble salts from gold metal in the presence of air:
- 4 Au + 8 KCN + O2 + 2 H2O → 4 K[Au(CN)2] + 4 KOH
Very few alternative methods exist for this extraction process.
KCN and the related NaCN are widely used in organic synthesis for the preparation of nitriles and carboxylic acids; illustrative in the von Richter reaction.
Toxicity
KCN can be detoxified most efficiently with hydrogen peroxide:[2]: KCN + H2O2 → KOCN + H2O
Cyanide is a potent inhibitor of cellular respiration, acting on mitochondrial cytochrome c oxidase and hence blocking oxidative phosphorylation. This prevents the body from oxidizing food to produce useful energy. Lactic acidosis then occurs as a consequence of anaerobic metabolism. Initially, acute cyanide poisoning causes a red or ruddy complexion in the victim because the tissues are not able to use the oxygen in the blood. The effects of potassium and sodium cyanide are identical. The person may die within 45 minutes if not treated medically. During this period, convulsions may occur. Death occurs mainly by cardiac arrest.
A number of prominent persons were killed or committed suicide using potassium cyanide, including members of the Black Hand Gang (unsuccessfully) and members of the Nazi Party, such as Hermann Göring and Heinrich Himmler, WWII era British agents (using purpose-made suicide pills), and various religious cults such as in Jonestown. Potassium cyanide (and other forms of cyanide) is a popular method of murder in fiction, especially in the books written by Agatha Christie.
References
- ↑ Bernard Martel. Chemical Risk Analysis: A Practical Handbook. Kogan, 2004, page 361. ISBN 1903996651.
- ↑ 2.0 2.1 2.2 Andreas Rubo, Raf Kellens, Jay Reddy, Joshua Wooten, Wolfgang Hasenpusch "Alkali Metal Cyanides" in Ullmann's Encyclopedia of Industrial Chemistry 2006 Wiley-VCH, Weinheim, Germany. doi:10.1002/14356007.i01_i01
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 304300
- ↑ H. T. Stokes, D. L. Decker, H. M. Nelson, J. D. Jorgensen (1993). "Structure of potassium cyanide at low temperature and high pressure determined by neutron diffraction". Physical Review B 47 (17): 11082–11092. doi:10.1103/PhysRevB.47.11082..
External links
- International Chemical Safety Card 0671
- Hydrogen cyanide and cyanides (CICAD 61)
- National Pollutant Inventory - Cyanide compounds fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- European Chemicals Bureau
- CSST (Canada)
- NIST Standard Reference Database
- Institut national de recherche et de sécurité (1997). "Cyanure de sodium. Cyanure de potassium". Fiche toxicologique n° 111, Paris:INRS, 6pp. (PDF file, in French)
|
|||||