Biofilm
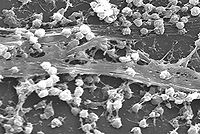
A biofilm is an aggregate of microorganisms in which cells adhere to each other and/or to a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). Biofilm EPS, which is also referred to as slime (although not everything described as slime is a biofilm), is a polymeric conglomeration generally composed of extracellular DNA, proteins, and polysaccharides in various configurations. Biofilms may form on living or non-living surfaces, and represent a prevalent mode of microbial life in natural, industrial and hospital settings.[1] The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single-cells that may float or swim in a liquid medium.
Microbes form a biofilm in response to many factors, which may include cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or in some cases, by exposure of planktonic cells to sub-inhibitory concentrations of antibiotics.[2][3] When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated.[4]
Contents |
Formation
Formation of a biofilm begins with the attachment of free-floating microorganisms to a surface. These first colonists adhere to the surface initially through weak, reversible van der Waals forces. If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion structures such as pili.[5]
The first colonists facilitate the arrival of other cells by providing more diverse adhesion sites and beginning to build the matrix that holds the biofilm together. Some species are not able to attach to a surface on their own but are often able to anchor themselves to the matrix or directly to earlier colonists. It is during this colonization that the cells are able to communicate via quorum sensing using such products as AHL. Once colonization has begun, the biofilm grows through a combination of cell division and recruitment. The final stage of biofilm formation is known as development, and is the stage in which the biofilm is established and may only change in shape and size. The development of a biofilm may allow for an aggregate cell colony (or colonies) to be increasingly antibiotic resistant.
Development
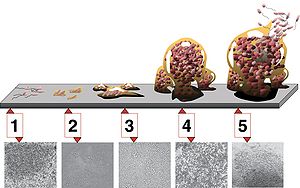
There are five stages of biofilm development (see illustration at right).
- initial attachment
- irreversible attachment
- maturation I
- maturation II
- dispersion
Dispersal
Dispersal of cells from the biofilm colony is an essential stage of the biofilm lifecycle. Dispersal enables biofilms to spread and colonize new surfaces. Enzymes that degrade the biofilm extracellular matrix, such as dispersin B and deoxyribonuclease, may play a role in biofilm dispersal.[6][7] Biofilm matrix degrading enzymes may be useful as anti-biofilm agents.[8][9] Recent evidence has shown that a fatty acid messenger, cis-2-decenoic acid, is capable of inducing dispersion and inhibiting growth of biofilm colonies. Secreted by Pseudomonas aeruginosa, this compound induces dispersion in several species of bacteria and the yeast Candida albicans[10]
Properties
Biofilms are usually found on solid substrates submerged in or exposed to some aqueous solution, although they can form as floating mats on liquid surfaces and also on the surface of leaves, particularly in high humidity climates. Given sufficient resources for growth, a biofilm will quickly grow to be macroscopic. Biofilms can contain many different types of microorganism, e.g. bacteria, archaea, protozoa, fungi and algae; each group performing specialized metabolic functions. However, some organisms will form monospecies films under certain conditions.
Researchers from the Helmholtz Center for Infection Research have investigated the strategies used by biofilms. They discovered that biofilm bacteria apply chemical weapons in order to defend themselves against disinfectants and antibiotics, phagocytes, and the immune systems of animals.
The lead researcher, Dr. Carsten Matz, began a serious investigation in order to find why phagocytes cannot annihilate the biofilm bacteria. He analyzed marine bacteria which defend themselves against amoebae, the behavior of which copies the behavior of phagocytes. The amoebae behave in the sea just like animal immune cells: they search for and feed on the bacteria. When bacteria are alone and separated in the water, they become easy prey for amoebae; however, when they attach to a surface and join other bacteria, amoebae cannot consume them.
The researcher stated that biofilms may be seen as a source of new bioactive agents. When bacteria are organized in biofilms they produce effective substances which individual bacteria are unable to produce alone.[11]
Extracellular matrix
The biofilm is held together and protected by a matrix of excreted polymeric compounds called EPS. EPS is an abbreviation for either extracellular polymeric substance or exopolysaccharide. This matrix protects the cells within it and facilitates communication among them through biochemical signals. Some biofilms have been found to contain water channels that help distribute nutrients and signalling molecules. This matrix is strong enough that under certain conditions, biofilms can become fossilized.
Bacteria living in a biofilm usually have significantly different properties from free-floating bacteria of the same species, as the dense and protected environment of the film allows them to cooperate and interact in various ways. One benefit of this environment is increased resistance to detergents and antibiotics, as the dense extracellular matrix and the outer layer of cells protect the interior of the community. In some cases antibiotic resistance can be increased a thousand-fold.[12]
The concept that biofilms are more resistant to antimicrobials is not completely accurate. For instance the biofilm form of Pseudomonas aeruginosa has no greater resistance to antimicrobials, when compared to stationary phase planktonic cells. Although, when the biofilm is compared to logarithmic phase planktonic cells, the biofilm does have greater resistance to antimicrobials. This resistance to antibiotics in both stationary phase cells and biofilms may be due to the presence of persister cells.[13]
Examples
Biofilms are ubiquitous. Nearly every species of microorganism, not only bacteria and archaea, have mechanisms by which they can adhere to surfaces and to each other. Biofilms will form on virtually every non-shedding surface in a non-sterile aqueous environment.
- Biofilms can be found on rocks and pebbles at the bottom of most streams or rivers and often form on the surface of stagnant pools of water. In fact, biofilms are important components of food chains in rivers and streams and are grazed by the aquatic invertebrates upon which many fish feed.
- Biofilms can grow in the most extreme environments: from, for example, the extremely hot, briny waters of hot springs ranging from very acidic to very alkaline, to frozen glaciers.
- In the human environment, biofilms can grow in showers very easily since they provide a moist and warm environment for the biofilm to thrive. Biofilms can form inside water and sewage pipes and cause clogging and corrosion. Biofilms on floors and counters can make sanitation difficult in food preparation areas.
- Biofilms in cooling-water systems are known to reduce heat transfer.[14]
- Biofilms in marine engineering systems, such as pipelines of the offshore oil and gas industry,[15] can lead to substantial corrosion problems. Corrosion is mainly due to abiotic factors; however, at least 20% is caused by microorganisms that are attached to the metal subsurface (i.e., microbially-influenced corrosion).
- Bacterial adhesion to boat hulls serves as the foundation for biofouling of seagoing vessels. Once a film of bacteria forms, it is easier for other marine organisms such as barnacles to attach. Such fouling can reduce maximum vessel speed by up to 20%, prolonging voyages and consuming fuel. Time in dry dock for refitting and repainting reduces the productivity of shipping assets, and the useful life of ships is also reduced due to corrosion and mechanical removal (scraping) of marine organisms from ships’ hulls.
- Biofilms can also be harnessed for constructive purposes. For example, many sewage treatment plants include a treatment stage in which waste water passes over biofilms grown on filters, which extract and digest organic compounds. In such biofilms, bacteria are mainly responsible for removal of organic matter (BOD); whilst protozoa and rotifers are mainly responsible for removal of suspended solids (SS), including pathogens and other microorganisms. Slow sand filters rely on biofilm development in the same way to filter surface water from lake, spring or river sources for drinking purposes. What we regard as clean water is a waste material to these microcellular organisms since they are unable to extract any further nutrition from the purified water.
- Biofilms can help eliminate petroleum oil from contaminated oceans or marine systems. The oil is eliminated by the hydrocarbon-degrading activities of microbial communities, in particular by a remarkable recently-discovered group of specialists, the so-called hydrocarbonoclastic bacteria (HCB).[16]
- Stromatolites are layered accretionary structures formed in shallow water by the trapping, binding and cementation of sedimentary grains by microbial biofilms, especially of cyanobacteria. Stromatolites include some of the most ancient records of life on Earth, and are still forming today.
- Biofilms are present on the teeth of most animals as dental plaque, where they may cause tooth decay and gum disease.
- Biofilms are found on the surface of and inside plants. They can either contribute to crop disease or, as in the case of nitrogen-fixing Rhizobium on roots, exist symbiotically with the plant [17]. Examples of crop diseases related to biofilms include Citrus Canker, Pierce's Disease of grapes, and Bacterial Spot of plants such as peppers and tomatoes.[18]
Biofilms and infectious diseases
Biofilms have been found to be involved in a wide variety of microbial infections in the body, by one estimate 80% of all infections.[19] Infectious processes in which biofilms have been implicated include common problems such as urinary tract infections, catheter infections, middle-ear infections, formation of dental plaque,[20] gingivitis,[20] coating contact lenses,[21] and less common but more lethal processes such as endocarditis, infections in cystic fibrosis, and infections of permanent indwelling devices such as joint prostheses and heart valves.[22][23] More recently it has been noted that bacterial biofilms may impair cutaneous wound healing and reduce topical antibacterial efficiency in healing or treating infected skin wounds.[24]
It has recently been shown that biofilms are present on the removed tissue of 80% of patients undergoing surgery for chronic sinusitis. The patients with biofilms were shown to have been denuded of cilia and goblet cells, unlike the controls without biofilms who had normal cilia and goblet cell morphology.[25] Biofilms were also found on samples from two of 10 healthy controls mentioned. The species of bacteria from interoperative cultures did not correspond to the bacteria species in the biofilm on the respective patient's tissue. In other words, the cultures were negative though the bacteria were present.[26]
Biofilms can also be formed on the inert surfaces of implanted devices such as catheters, prosthetic cardiac valves and intrauterine devices. [27]
New staining techniques are being developed to differentiate bacterial cells growing in living animals, e.g. from tissues with allergy-inflammations .[28]
Pseudomonas aeruginosa biofilms
The achievements of medical care in industrialised societies are markedly impaired due to chronic opportunistic infections that have become increasingly apparent in immunocompromised patients and the aging population. Chronic infections remain a major challenge for the medical profession and are of great economic relevance because traditional antibiotic therapy is usually not sufficient to eradicate these infections. One major reason for persistence seems to be the capability of the bacteria to grow within biofilms that protects them from adverse environmental factors. Pseudomonas aeruginosa is not only an important opportunistic pathogen and causative agent of emerging nosocomial infections but can also be considered a model organism for the study of diverse bacterial mechanisms that contribute to bacterial persistence. In this context the elucidation of the molecular mechanisms responsible for the switch from planktonic growth to a biofilm phenotype and the role of inter-bacterial communication in persistent disease should provide new insights in P. aeruginosa pathogenicity, contribute to a better clinical management of chronically infected patients and should lead to the identification of new drug targets for the development of alternative anti-infective treatment strategies.[29]
Dental plaque
Dental plaque is the material that adheres to the teeth and consists of bacterial cells (mainly Streptococcus mutans and Streptococcus sanguinis), salivary polymers and bacterial extracellular products. Plaque is a biofilm on the surfaces of the teeth. This accumulation of microorganisms subject the teeth and gingival tissues to high concentrations of bacterial metabolites which results in dental disease.[20]
Legionellosis
Legionella bacteria are known to grow under certain conditions in biofilms, in which they are protected against disinfectants. Workers in cooling towers, persons working in air conditioned rooms and people taking a shower are exposed to Legionella by inhalation when the systems are not well designed, constructed, or maintained.[30]
Neisseria gonorrhoeae biofilms
Neisseria gonorrhoeae is an exclusive human pathogen. Recent studies have demonstrated that it utilizes two distinct mechanisms for entry into human urethral and cervical epithelial cells involving different bacterial surface ligands and host receptors. In addition it has been demonstrated that the gonococcus can form biofilms on glass surfaces and over human cells. There is evidence for formation of gonococcal biofilms on human cervical epithelial cells during natural disease and that outer membrane blebbing by the gonococcus is crucial in biofilm formation over human cervical epithelial cells.[31]
Molecular genetics
Technological progress in microscopy, molecular genetics and genome analysis has significantly advanced our understanding of the structural and molecular aspects of biofilms, especially of extensively studied model organisms such as Pseudomonas aeruginosa. Biofilm development can be divided into several key steps including attachment, microcolony formation, biofilm maturation and dispersion; and in each step bacteria may recruit different components and molecules including flagellae, type IV pili, DNA and exopolysaccharides.[32][33] The rapid progress in biofilm research has also unveiled several genetic regulation mechanisms implicated in biofilm regulation such as quorum sensing and the novel secondary messenger cyclic-di-GMP. Understanding the molecular mechanisms of biofilm formation has facilitated the exploration of novel strategies to control bacterial biofilms.[34]
See also
- Kombucha
- Microbial mat
- Phototrophic biofilms
- Stromatolite
References
- Allison, D. G. (2000). Community structure and co-operation in biofilms. Cambridge, UK: Cambridge University Press. ISBN 0-521-79302-5.
- Lynch, James F.; Lappin-Scott, Hilary M.; Costerton, J. W. (2003). Microbial biofilms. Cambridge, UK: Cambridge University Press. ISBN 0-521-54212-X.
- "A Friendly Guide to Biofilm Basics & the CBE". Center for Biofilm Engineering, Montana State University. http://www.erc.montana.edu/CBEssentials-SW/bf-basics-99/default.htm.
- Fratamico, M. (2009). Biofilms in the food and beverage industries. Woodhead Publishing Limited. ISBN 978-1-84569-477-7.
Footnotes
- ↑ Hall-Stoodley L, Costerton JW, Stoodley P (February 2004). "Bacterial biofilms: from the natural environment to infectious diseases". Nature Reviews. Microbiology 2 (2): 95–108. doi:10.1038/nrmicro821. PMID 15040259.
- ↑ Karatan E, Watnick P (June 2009). "Signals, regulatory networks, and materials that build and break bacterial biofilms". Microbiology and Molecular Biology Reviews 73 (2): 310–47. doi:10.1128/MMBR.00041-08. PMID 19487730. PMC 2698413. http://mmbr.asm.org/cgi/pmidlookup?view=long&pmid=19487730.
- ↑ Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (August 2005). "Aminoglycoside antibiotics induce bacterial biofilm formation". Nature 436 (7054): 1171–5. doi:10.1038/nature03912. PMID 16121184. (primary source)
- ↑ An D, Parsek MR (June 2007). "The promise and peril of transcriptional profiling in biofilm communities". Current Opinion in Microbiology 10 (3): 292–6. doi:10.1016/j.mib.2007.05.011. PMID 17573234.
- ↑ JPG Images: niaid.nih.gov erc.montana.edu
- ↑ Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (August 2003). "Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity". Journal of Bacteriology 185 (16): 4693–8. doi:10.1128/JB.185.16.4693-4698.2003. PMID 12896987.
- ↑ Izano EA, Amarante MA, Kher WB, Kaplan JB (January 2008). "Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms". Applied and Environmental Microbiology 74 (2): 470–6. doi:10.1128/AEM.02073-07. PMID 18039822.
- ↑ Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N (July 2004). "Enzymatic detachment of Staphylococcus epidermidis biofilms". Antimicrobial Agents and Chemotherapy 48 (7): 2633–6. doi:10.1128/AAC.48.7.2633-2636.2004. PMID 15215120.
- ↑ Xavier JB, Picioreanu C, Rani SA, van Loosdrecht MC, Stewart PS (December 2005). "Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix--a modelling study". Microbiology 151 (Pt 12): 3817–32. doi:10.1099/mic.0.28165-0. PMID 16339929.
- ↑ Davies DG, Marques CN (March 2009). "A fatty acid messenger is responsible for inducing dispersion in microbial biofilms". Journal of Bacteriology 191 (5): 1393–403. doi:10.1128/JB.01214-08. PMID 19074399.
- ↑ Biofilm Bacteria Protect Themselves With Chemical Weapons
- ↑ Stewart PS, Costerton JW (July 2001). "Antibiotic resistance of bacteria in biofilms". Lancet 358 (9276): 135–8. doi:10.1016/S0140-6736(01)05321-1. PMID 11463434.
- ↑ Spoering AL, Lewis K (December 2001). "Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials". Journal of Bacteriology 183 (23): 6746–51. doi:10.1128/JB.183.23.6746-6751.2001. PMID 11698361.
- ↑ Characklis, W. G.; Nevimons, M. J.; Picologlou, B. F. (1981). "Influence of Fouling Biofilms on Heat Transfer". Heat Transfer Engineering 3: 23. doi:10.1080/01457638108939572.
- ↑ Schwermer CU, Lavik G, Abed RM, et al. (May 2008). "Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields". Applied and Environmental Microbiology 74 (9): 2841–51. doi:10.1128/AEM.02027-07. PMID 18344353.
- ↑ Martins VAP et al. (2008). "Genomic Insights into Oil Biodegradation in Marine Systems". Microbial Biodegradation: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-17-2. http://www.horizonpress.com/biod.
- ↑ Introduction to Biofilms: Desirable and undesirable impacts of biofilm
- ↑ Andersen PC, Brodbeck BV, Oden S, Shriner A, Leite B (September 2007). "Influence of xylem fluid chemistry on planktonic growth, biofilm formation and aggregation of Xylella fastidiosa". FEMS Microbiology Letters 274 (2): 210–7. doi:10.1111/j.1574-6968.2007.00827.x. PMID 17610515.
- ↑ "Research on microbial biofilms (PA-03-047)". NIH, National Heart, Lung, and Blood Institute. 2002-12-20. http://grants.nih.gov/grants/guide/pa-files/PA-03-047.html.
- ↑ 20.0 20.1 20.2 Rogers A H (2008). Molecular Oral Microbiology. Caister Academic Press. ISBN 978-1-904455-24-0. http://www.horizonpress.com/oral2.
- ↑ Imamura Y, Chandra J, Mukherjee PK, et al. (January 2008). "Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions". Antimicrobial Agents and Chemotherapy 52 (1): 171–82. doi:10.1128/AAC.00387-07. PMID 17999966.
- ↑ Lewis K (April 2001). "Riddle of biofilm resistance". Antimicrobial Agents and Chemotherapy 45 (4): 999–1007. doi:10.1128/AAC.45.4.999-1007.2001. PMID 11257008.
- ↑ Parsek MR, Singh PK (2003). "Bacterial biofilms: an emerging link to disease pathogenesis". Annual Review of Microbiology 57: 677–701. doi:10.1146/annurev.micro.57.030502.090720. PMID 14527295.
- ↑ Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM (2008). "Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo". Wound Repair and Regeneration 16 (1): 23–9. doi:10.1111/j.1524-475X.2007.00303.x. PMID 18211576.
- ↑ Sanclement J, Webster P, Thomas J, Ramadan H (2005). "Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis". Laryngoscope 115 (4): 578–82. doi:10.1097/01.mlg.0000161346.30752.18 (inactive 2010-02-01). PMID 15805862.
- ↑ Sanderson AR, Leid JG, Hunsaker D (July 2006). "Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis". The Laryngoscope 116 (7): 1121–6. doi:10.1097/01.mlg.0000221954.05467.54. PMID 16826045.
- ↑ Auler ME, Morreira D, Rodrigues FF, et al. (April 2009). "Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis". Medical Mycology: 1–6. doi:10.1080/13693780902856626. PMID 19353374.
- ↑ Leevy WM, Gammon ST, Jiang H, et al. (December 2006). "Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe". Journal of the American Chemical Society 128 (51): 16476–7. doi:10.1021/ja0665592. PMID 17177377.
- ↑ Cornelis P (2008). Pseudomonas: Genomics and Molecular Biology (1st ed.). Caister Academic Press. ISBN 978-1-904455-19-6. http://www.horizonpress.com/pseudo.
- ↑ Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM (November 2001). "Role of biofilms in the survival of Legionella pneumophila in a model potable-water system". Microbiology 147 (Pt 11): 3121–6. PMID 11700362.
- ↑ Apicella M et al. (2010). "Gonococcal Biofilms". Neisseria: Molecular Mechanisms of Pathogenesis. Caister Academic Press. ISBN 978-1-904455-51-6.
- ↑ Jarrell K (2009). Pili and Flagella: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-48-6.
- ↑ Ullrich M (2009). Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.
- ↑ An S et al. (2010). "The Impact and Molecular Genetics of Bacterial Biofilms". Environmental Molecular Microbiology. Caister Academic Press. ISBN 978-1-904455-52-3.
Further reading
- Ramadan HH, Sanclement JA, Thomas JG (March 2005). "Chronic rhinosinusitis and biofilms". Otolaryngology--Head and Neck Surgery 132 (3): 414–7. doi:10.1016/j.otohns.2004.11.011. PMID 15746854.
- Bendouah Z, Barbeau J, Hamad WA, Desrosiers M (June 2006). "Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis". Otolaryngology--Head and Neck Surgery 134 (6): 991–6. doi:10.1016/j.otohns.2006.03.001. PMID 16730544.
- Lynch AS, Robertson GT (2008). "Bacterial and fungal biofilm infections". Annual Review of Medicine 59: 415–28. doi:10.1146/annurev.med.59.110106.132000. PMID 17937586.
- Vo P, Nunez M (2010). "Bdellovibrio bacteriovorus Predation in Dual-Species Biofilms of E. coli Prey and M. luteus Decoys". ArXiv. link