Gluconeogenesis
Gluconeogenesis (abbreviated GNG) is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids.
It is one of the two main mechanisms the body uses to keep blood glucose levels from dropping too low (hypoglycemia). The other means of maintaining blood glucose levels is through the degradation of glycogen (glycogenolysis). [1]
Gluconeogenesis is a ubiquitous process, present in plants, animals, fungi, and other microorganisms.[2] In animals, gluconeogenesis takes place mainly in the liver and, to a smaller extent, in the cortex of kidneys. This process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise and is highly endergonic. Gluconeogenesis is often associated with ketosis. Gluconeogenesis is also a target of therapy for type II diabetes, such as metformin, which inhibits glucose formation and stimulates glucose uptake by cells.[3]
Contents |
Entering the pathway
Lactate is transported back to the liver where it is converted into pyruvate by the Cori cycle using the enzyme lactate dehydrogenase. Pyruvate, the first designated substrate of the gluconeogenic pathway, can then be used to generate glucose.[4] All citric acid cycle intermediates, through conversion to oxaloacetate, amino acids other than lysine or leucine, and glycerol can also function as substrates for gluconeogenesis.[4] Transamination or deamination of amino acids facilitates entering of their carbon skeleton into the cycle directly (as pyruvate or oxaloacetate), or indirectly via the citric acid cycle.
Fatty acids cannot be converted into glucose in animals with the exception of odd-chain fatty acids, which yield propionyl CoA, a precursor for succinyl CoA. In plants, specifically in seedlings, the glyoxylate cycle can be used to convert fatty acids (acetate) into the primary carbon source of the organism. The glyoxylate cycle produces four-carbon dicarboxylic acids that can enter gluconeogenesis.[4] Glycerol, which is a part of all triacylglycerols, can also be used in gluconeogenesis.
Pathway
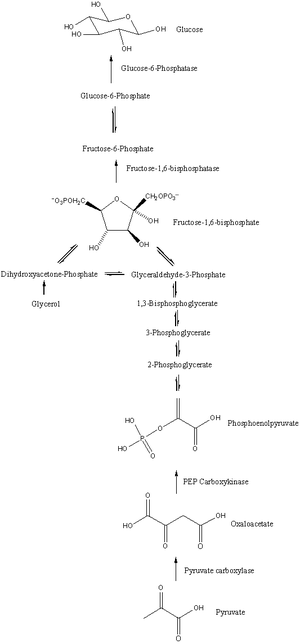
Gluconeogenesis is a pathway consisting of eleven enzyme-catalyzed reactions. The pathway can begin in the mitochondria or cytoplasm, depending on the substrate being used. Many of the reactions are the reversible steps found in glycolysis.
- Gluconeogenesis begins in the mitochondria with the formation of oxaloacetate through carboxylation of pyruvate. This reaction also requires one molecule of ATP, and is catalyzed by pyruvate carboxylase. This enzyme is stimulated by high levels of acetyl-CoA (produced in β-oxidation in the liver) and inhibited by high levels of ADP.
- Oxaloacetate is reduced to malate using NADH, a step required for transport out of the mitochondria.
- Malate is oxidized to oxaloacetate using NAD+ in the cytoplasm, where the remaining steps of gluconeogenesis occur.
- Oxaloacetate is decarboxylated and phosphorylated to produce phosphoenolpyruvate by phosphoenolpyruvate carboxykinase. One molecule of GTP is hydrolyzed to GDP during this reaction.
- The next steps in the reaction are the same as reversed glycolysis. However, fructose-1,6-bisphosphatase converts fructose-1,6-bisphosphate to fructose 6-phosphate, requiring one water molecule and releasing one phosphate. This is also the rate limiting step of gluconeogenesis.
- Glucose-6-phosphate is formed from fructose 6-phosphate by phosphoglucoisomerase. Glucose-6-phosphate can be used in other metabolic pathways or dephosphorylated to free glucose. Whereas free glucose can easily diffuse in and out of the cell, the phosphorylated form (glucose-6-phosphate) is locked in the cell, a mechanism by which intracellular glucose levels are controlled by cells.
- The final reaction of gluconeogenesis, the formation of glucose, occurs in the lumen of the endoplasmic reticulum where glucose-6-phosphate is hydrolyzed by glucose-6-phosphatase to produce glucose. Glucose is shuttled into the cytosol by glucose transporters located in the membrane of the endoplasmic reticulum.
Regulation
While most steps in gluconeogenesis are the reverse of those found in glycolysis, three regulated and strongly exergonic reactions are replaced with more kinetically favorable reactions. Hexokinase/glucokinase, phosphofructokinase, and pyruvate kinase enzymes of glycolysis are replaced with glucose-6-phosphatase, fructose-1,6-bisphosphatase, and PEP carboxykinase. This system of reciprocal control allow glycolysis and gluconeogenesis to inhibit each other and prevent the formation of a futile cycle.
The majority of the enzymes responsible for gluconeogenesis are found in the cytoplasm; the exceptions are mitochondrial pyruvate carboxylase, and, in animals, phosphoenolpyruvate carboxykinase. The latter exists as an isozyme located in both the mitochondrion and the cytosol.[5] The rate of gluconeogenesis is ultimately controlled by the action of a key enzyme, fructose-1,6-bisphosphatase, which is also regulated through signal tranduction by cAMP and its phosphorylation.
Most factors that regulate the activity of the gluconeogenesis pathway do so by inhibiting the activity or expression of key enzymes. However, both acetyl CoA and citrate activate gluconeogenesis enzymes (pyruvate carboxylase and fructose-1,6-bisphosphatase, respectively). Due to the reciprocal control of the cycle, acetyl-CoA and citrate also have inhibitory roles in the activity of pyruvate kinase.
References
- ↑ Silva, Pedro. "The Chemical Logic Behind Gluconeogenesis". http://www2.ufp.pt/~pedros/bq/gng.htm. Retrieved September 8, 2009.
- ↑ David L Nelson and Michael M Cox (2000). Lehninger Principles of Biochemistry. USA: Worth Publishers. pp. 724. ISBN 1-57259-153-6.
- ↑ Hundal R, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi S, Schumann W, Petersen K, Landau B, Shulman G (2000). "Mechanism by which metformin reduces glucose production in type 2 diabetes". Diabetes 49 (12): 2063–9. doi:10.2337/diabetes.49.12.2063. PMID 11118008. Free full textPDF (82 KiB)
- ↑ 4.0 4.1 4.2 Garrett, Reginald H.; Charles M. Grisham (2002). Principles of Biochemistry with a Human Focus. USA: Brooks/Cole, Thomson Learning. pp. 578,585. ISBN 0-03-097369-4.
- ↑ Chakravarty, K., Cassuto, H., Resef, L., & Hanson, R.W. (2005) Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Critical Reviews of Biochemistry and Molecular Biology, 40(3), 129-154.
External links
- Overview at indstate.edu
- Interactive diagram at uakron.edu
- The chemical logic behind gluconeogenesis
|
|||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||
|
|||||||||||