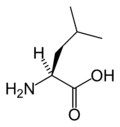
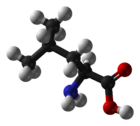
Leucine
| Leucine | |
|---|---|
 |
 |
|
Leucine
|
|
|
Other names
2-Amino-4-methylpentanoic acid
|
|
| Identifiers | |
| CAS number | 61-90-5 |
| PubChem | 6106 |
|
SMILES
CC(C)C[C@H](N)C(O)=O
|
|
| Properties | |
| Molecular formula | C6H13NO2 |
| Molar mass | 131.17 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Leucine (abbreviated as Leu or L)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH(CH3)2. It is an essential amino acid, which means that humans cannot synthesise it. Its codons are UUA, UUG, CUU, CUC, CUA, and CUG. With a hydrocarbon side chain, leucine is classified as a hydrophobic amino acid. It has an isobutyl R group. Leucine is a major component of the sub units in ferritin, astacin and other 'buffer' proteins.
Contents |
Biosynthesis
As an essential amino acid, leucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. It is synthesized in plants and microorganisms via several steps starting from pyruvic acid. The initial part of the pathway also leads to valine. The intermediate α-ketovalerate is converted to α-isopropylmalate and then β-isopropylmalate, which is dehydrogenated to α-ketoisocaproate, which in the final step undergoes reductive amination. Enzymes involved in a typical leucine biosynthesis include[2]
- Acetolactate synthase,
- Acetohydroxy acid isomeroreductase,
- Dihydroxyacid dehydratase,
- α-Isopropylmalate synthase,
- α-Isopropylmalate isomerase,
- Leucine aminotransferase.
Biology
As a dietary supplement, leucine has been found to slow the degradation of muscle tissue by increasing the synthesis of muscle proteins in aged rats.[3] Leucine is utilized in the liver, adipose tissue, and muscle tissue. In adipose and muscle tissue, leucine is used in the formation of sterols, and the combined usage of leucine in these two tissues is seven times greater than its use in the liver.[4]
Leucine toxicity, as seen in decompensated Maple Syrup Urine Disease (MSUD), causes delirium and neurologic compromise, and can be life-threatening.
In yeast genetics, mutants with a defective gene for leucine synthesis (leu2) are transformed with a plasmid that contains a working leucine synthesis gene (LEU2) and grown on minimal media. Leucine synthesis then becomes a useful selectable marker.
Dietary aspects
| Food | g/100g |
|---|---|
| Soy protein concentrate | 4.917 |
| Peanuts | 1.672 |
| Wheat germ | 1.571 |
| Almonds | 1.488 |
| Oat | 1.284 |
| Beans, pinto, cooked | 0.765 |
| Lentils, cooked | 0.654 |
| Chickpea, cooked | 0.631 |
| Corn, yellow | 0.348 |
| Rice, brown, medium-grain, cooked | 0.191 |
Betaines

Chemical properties
Leucine is a branched-chain amino acid (BCAA) since it possesses an aliphatic side-chain that is non-linear.
Racemic leucine had been subjected to circularly polarized synchrotron radiation in order to better understand the origin of biomolecular asymmetry. An enantiomeric enhancement of 2.6 % had been induced, indicating a photochemical origin of biomolecules' homochirality.[6]
Food additive
As a food additive, L-Leucine has E number E641 and is classified as a flavour enhancer.
See also
- Leucines, description of the isomers of leucine
- Photo-reactive leucine
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. http://www.chem.qmul.ac.uk/iupac/AminoAcid/. Retrieved 2007-05-17.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ L. Combaret, et al. Human Nutrition Research Centre of Clermont-Ferrand. "A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle". Journal of Physiology Volume 569, issue 2, p. 489-499. http://jp.physoc.org/cgi/reprint/569/2/489. Retrieved 2008-03-25.
- ↑ J. Rosenthal, et al. Department of Medicine, University of Toronto, Toronto, Canada. "Metabolic fate of leucine: A significant sterol precursor in adipose tissue and muscle". American Journal of Physiology Vol. 226, No. 2, p. 411-418. http://ajplegacy.physiology.org/cgi/reprint/226/2/411. Retrieved 2008-03-25.
- ↑ National Nutrient Database for Standard Reference, U.S. Department of Agriculture, http://www.nal.usda.gov/fnic/foodcomp/search/, retrieved 2009-09-16.
- ↑ Meierhenrich: Amino acids and the asymmetry of life, Springer-Verlag, 2008, ISBN 978-3-540-76885-2
External links
- Food Sources of Leucine
- Leucine biosynthesis
- Leucine content in food
- Leucine prevents muscle loss in rats
- Leucine helps regulate appetite in rats
- Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects
- A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle
|
||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||