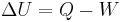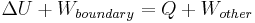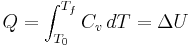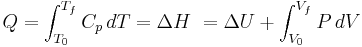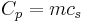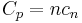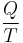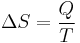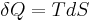Heat
In physics and thermodynamics, heat is the process of energy transfer from one body or system to another due to thermal contact, which in turn is defined as an energy transfer to a body in any other way than due to work performed on the body.[1]
A related term is thermal energy, loosely defined as the energy of a body that increases with its temperature. Heat is also loosely referred to as thermal energy, although many definitions require this thermal energy to actually be in the process of movement between one body and another to be technically called heat (otherwise, many sources prefer to continue to refer to the static quantity as "thermal energy"). Heat is a means of energy transfer, rather than a form of energy. Energy is transferred, not heat.
Energy transfer by heat can occur between objects by radiation, conduction and convection. Temperature is used as a measure of the internal energy or enthalpy, that is the level of elementary motion giving rise to heat transfer. Energy can only be transferred by heat between objects - or areas within an object - with different temperatures (as given by the zeroth law of thermodynamics). This transfer happens spontaneously only in the direction of the colder body (as per the second law of thermodynamics). The transfer of energy by heat from one object to another object with an equal or higher temperature can happen only with the aid of a heat pump via mechanical work.
Contents |
Overview
In modern terms, heat is defined as energy in transit. Scottish physicist James Clerk Maxwell, in his 1871 classic Theory of Heat, was one of the first to enunciate a modern definition of “heat”. Maxwell outlined four stipulations on the definition of heat. One, it is “something which may be transferred from one body to another”, as per the second law of thermodynamics. Two, it is a “measurable quantity”, and thus treated mathematically. Three, it “can not be treated as a substance”; for it may be transformed into something which is not a substance, e.g. mechanical work. Lastly, it is “one of the forms of energy”.
Heat flows between systems that are not in thermal equilibrium with each other; it spontaneously flows from the areas of high temperature to areas of low temperature. When two bodies of different temperature come into thermal contact, they will exchange internal energy until their temperatures are equalized; that is, until they reach thermal equilibrium.
The first law of thermodynamics states that the energy of an isolated system is conserved. Therefore, to change the energy of a system, energy must be transferred to or from the system. Heat and work are the only two mechanisms by which energy can be transferred. Work performed on a body is, by definition [1], an energy transfer to the body that is due to a change to external parameters of the body (such as the volume, magnetization, center of mass in a gravitational field, etc.). Heat is the energy transferred to the body in any other way.
In the case of bodies close to thermal equilibrium where notions such as the temperature can be defined, heat transfer can be related to temperature difference between bodies. It is an irreversible process that leads to the bodies coming closer to mutual thermal equilibrium.
Adjectives such as "hot" and "cold" are relative terms and are generally used to compare one object’s temperature to another or it's surroundings.
Definitions
Several modern definitions of heat are as follows:
- The energy going from a high-temperature object to a lower-temperature object is called heat.[2]
- The noun heat is defined only during the process of energy transfer by conduction or radiation.[3]
- Any spontaneous flow of energy from one object to another caused by a difference in temperature between the objects is called heat.[4]
As for its relationship with kinetic energy:
- The energy inside a substance is due to the kinetic energy of molecules or atoms.[5] The kinetic energy and heat may formally be equivalent, but they are not identical.
- In a thermodynamic sense, heat is never regarded as being stored within a body. Like work, it exists only as energy in transit from one body to another; in thermodynamic terminology, between a system and its surroundings. When energy in the form of heat is added to a system, it is stored not as heat, but as kinetic and potential energy of the atoms and molecules making up the system.[6]
According to some definitions, heat can flow in the absence of any temperature difference:
- The energy going between the two systems is called heat. However, an interaction between two closed systems that are initially isolated and in a stable equilibrium cannot do work on each other, so they only undergo pure heat (non-working) interactions.[7]
Notation and units
The unit for the amount of energy transferred by heat in the International System of Units SI is the joule (J), though theBritish Thermal Unit and the calorie are still used in the United States. The unit for the rate of heat transfer is the watt (W = J/s).
The total amount of energy transferred through heat transfer is conventionally abbreviated as Q. The conventional sign convention is that when a body releases heat into its surroundings, Q < 0 (-); when a body absorbs heat from its surroundings, Q > 0 (+). Heat transfer rate, or heat flow per unit time, is denoted by:
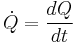 .
.
It is measured in watts. Heat flux is defined as rate of heat transfer per unit cross-sectional area, and is denoted q, resulting in units of watts per square metre, though slightly different notation conventions can be used.
Internal energy
Heat is related to the internal energy 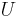 of the system and work
of the system and work 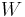 done by the system by the first law of thermodynamics:
done by the system by the first law of thermodynamics:
which means that the energy of the system can change either via work or via heat flows across the boundary of the thermodynamic system. In more detail, Internal energy is the sum of all microscopic forms of energy of a system. It is related to the molecular structure and the degree of molecular activity and may be viewed as the sum of kinetic and potential energies of the molecules; it comprises the following types of energies:[8]
| Type | Composition of Internal Energy (U) |
|---|---|
| Sensible energy | the portion of the internal energy of a system associated with kinetic energies (molecular translation, rotation, and vibration; electron translation and spin; and nuclear spin) of the molecules. |
| Latent energy | the internal energy associated with the phase of a system. |
| Chemical energy | the internal energy associated with the atomic bonds in a molecule. |
| Nuclear energy | the tremendous amount of energy associated with the strong bonds within the nucleus of the atom itself. |
| Energy interactions | those types of energies not stored in the system (e.g. heat transfer, mass transfer, and work), but which are recognized at the system boundary as they cross it, which represent gains or losses by a system during a process. |
| Thermal energy | the sum of sensible and latent forms of internal energy. |
The transfer of heat to an ideal gas at constant pressure increases the internal energy and performs boundary work (i.e. allows a control volume of gas to become larger or smaller), provided the volume is not constrained. Returning to the first law equation and separating the work term into two types, "boundary work" and "other" (e.g. shaft work performed by a compressor fan), yields the following:
This combined quantity 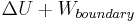 is enthalpy,
is enthalpy, 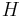 , one of the thermodynamic potentials. Both enthalpy,
, one of the thermodynamic potentials. Both enthalpy, 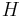 , and internal energy,
, and internal energy, 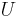 are state functions. State functions return to their initial values upon completion of each cycle in cyclic processes such as that of a heat engine. In contrast, neither
are state functions. State functions return to their initial values upon completion of each cycle in cyclic processes such as that of a heat engine. In contrast, neither 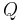 nor
nor 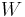 are properties of a system and need not sum to zero over the steps of a cycle. The infinitesimal expression for heat,
are properties of a system and need not sum to zero over the steps of a cycle. The infinitesimal expression for heat, 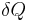 , forms an inexact differential for processes involving work. However, for processes involving no change in volume, applied magnetic field, or other external parameters,
, forms an inexact differential for processes involving work. However, for processes involving no change in volume, applied magnetic field, or other external parameters, 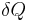 forms an exact differential. Likewise, for adiabatic processes (no heat transfer), the expression for work forms an exact differential, but for processes involving transfer of heat it forms an inexact differential.
forms an exact differential. Likewise, for adiabatic processes (no heat transfer), the expression for work forms an exact differential, but for processes involving transfer of heat it forms an inexact differential.
Enthalpy and internal energy changes
Ideal gas
For a simple compressible system such as an ideal gas inside a piston, the changes in enthalpy and internal energy can be related to the heat capacity at constant pressure and volume, respectively. Constrained to have constant volume, the heat, 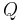 , required to change its temperature from an initial temperature, T0, to a final temperature, Tf is given by:
, required to change its temperature from an initial temperature, T0, to a final temperature, Tf is given by:
Removing the volume constraint and allowing the system to expand or contract at constant pressure:
Incompressible substances
For incompressible substances, such as solids and liquids, the distinction between the two types of heat capacity (i.e. 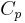 which is based on constant pressure and
which is based on constant pressure and 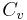 which is based on constant volume) disappears, as no work is performed.
which is based on constant volume) disappears, as no work is performed.
Specific heat
Specific heat is defined as the amount of energy that has to be transferred to or from one unit of mass or mole of a substance to change its temperature by one degree. Specific heat is a property, which means that it depends on the substance under consideration and its state as specified by its properties. Fuels, when burned, are converted to molecules with a lower internal energy. The change in energy is heat. Upon changing from one phase to another, a pure substance releases or absorbs heat without its temperature changing. The amount of heat transfer during a phase change is known as latent heat and depends primarily on the substance and its state.
The specific heats of monatomic gases (e.g., helium) are nearly constant with temperature. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more.
In liquids at sufficiently low temperatures, quantum effects become significant. An example is the behavior of bosons such as helium-4. For such substances, the behavior of heat capacity with temperature is discontinuous at the Bose-Einstein condensation point.
The quantum behavior of solids is adequately characterized by the Debye model. At temperatures well below the characteristic Debye temperature of a solid lattice, its specific heat will be proportional to the cube of absolute temperature. For low-temperature metals, a second term is needed to account for the behavior of the conduction electrons, an example of Fermi-Dirac statistics.
Calculating heat capacity from molar and specific heat capacity
The molar and specific heat capacities are dependent upon the internal degrees of freedom of the system and not on any bulk properties such as volume and number of molecules.
In contrast, heat capacity itself is an extensive quantity and as such is dependent on the number of molecules in the system. It can be represented as the product of mass, 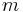 , and specific heat capacity,
, and specific heat capacity, 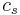 according to:
according to:
or is dependent on the number of moles and the molar heat capacity, 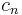 according to:
according to:
Entropy
In 1856, German physicist Rudolf Clausius defined the second fundamental theorem (the second law of thermodynamics) in the mechanical theory of heat (thermodynamics): "if two transformations which, without necessitating any other permanent change, can mutually replace one another, be called equivalent, then the generations of the quantity of heat Q from work at the temperature T, has theequivalence-value:"[9][10]
In 1865, he came to define this ratio as entropy symbolized by S, such that, for a closed, stationary system:
and thus, by reduction, quantities of heat δQ (an inexact differential) are defined as quantities of TdS (an exact differential):
In other words, the entropy function S facilitates the quantification and measurement of heat flow through a thermodynamic boundary.
Heat transfer in engineering

The discipline of heat transfer, typically considered an aspect of mechanical engineering and chemical engineering, deals with specific applied methods by which heat transfer occurs. Note that although the definition of "heat" implicitly means the movement of energy, the term "heat transfer" has acquired this traditional usage in engineering and other contexts. The understanding oh heat transfer is crucial for the design and operation of numerous devices and processes.
Heat transfer may occur by the mechanisms of conduction, radiation, and mass transfer. In engineering, the term convective heat transfer is used to describe the combined effects of conduction and fluid flow and is often regarded as an additional mechanism of heat transfer. Although separate physical laws have been discovered to describe the behaviour of each of these methods, real systems may exhibit a complicated combination. Various mathematical methods have been developed to solve or approximate the results of heat transfer in systems.
See also
|
|
References
- ↑ 1.0 1.1 F. Reif (2000). Fundamentals of Statistical and Thermal Physics. Singapore: McGraw-Hll, Inc.. p. 66. ISBN 0-07-Y85615-X.
- ↑ Discourse on Heat and Work - Department of Physics and Astronomy, Georgia State University: Hyperphysics (online)
- ↑ Baierlein, Ralph (2003). Thermal Physics. Cambridge University Press. ISBN 0521658381.
- ↑ Schroeder, Daniel V. (2000). An introduction to thermal physics. San Francisco, California: Addison-Wesley. p. 18. ISBN 0-321-27779-1. "Heat is defined as any spontaneous flow of energy from one object to another, caused by a difference in temperature between the objects."
- ↑ Clark, John, O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0760746168.
- ↑ Smith, J.M., Van Ness, H.C., Abbot, M.M. (2005). Introduction to Chemical Engineering Thermodynamics. McGraw-Hill. ISBN 0073104450.
- ↑ Perrot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0198565526.
- ↑ Cengel, Yungus, A.; Boles, Michael (2002). Thermodynamics: An Engineering Approach (4th ed.). Boston: McGraw-Hill. pp. 17–18. ISBN 0-07-238332-1.
- ↑ Published in Poggendoff’s Annalen, Dec. 1854, vol. xciii. p. 481; translated in the Journal de Mathematiques, vol. xx. Paris, 1855, and in the Philosophical Magazine, August 1856, s. 4. vol. xii, p. 81
- ↑ Clausius, R. (1865). The Mechanical Theory of Heat] –with its Applications to the Steam Engine and to Physical Properties of Bodies. London: John van Voorst, 1 Paternoster Row. MDCCCLXVII.
External links
- Plasma heat at 2 gigakelvins - Article about extremely high temperature generated by scientists (Foxnews.com)
- Correlations for Convective Heat Transfer - ChE Online Resources
- An Introduction to the Quantitative Definition and Analysis of Heat written for High School Students