Purine
| Purine | |
|---|---|
 |
|
|
7H-purine
|
|
| Identifiers | |
| CAS number | 120-73-0 |
| PubChem | 1044 |
| ChemSpider | 1015 |
| MeSH | Purine |
|
SMILES
C1=C2C(=NC=N1)N=CN2
|
|
| Properties | |
| Molecular formula | C5H4N4 |
| Molar mass | 120.11 g mol−1 |
| Melting point |
214 °C, 487 K, 417 °F |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
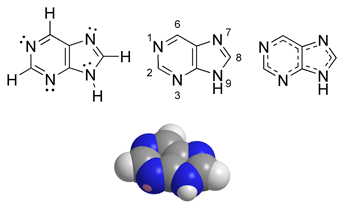
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature.[1]
Purines and pyrimidines make up the two groups of nitrogenous bases, including the two groups of nucleotide bases. Two of the four deoxyribonucleotides and two of the four ribonucleotides, the respective building blocks of DNA and RNA, are purines.
Contents |
Notable purines
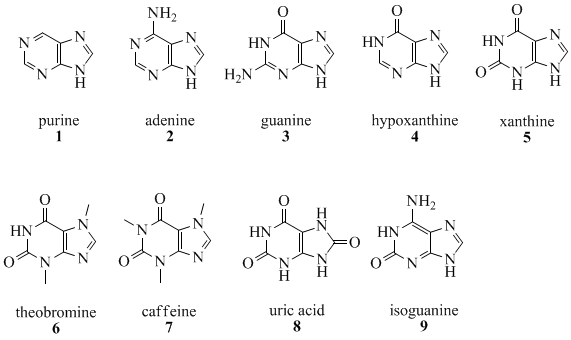
The quantity of naturally occurring purines produced on earth is huge. Fifty percent (50%) of the bases in nucleic acids, adenine (2) and guanine (3), are purines. In DNA, these bases form hydrogen bonds with their complementary pyrimidines thymine and cytosine, respectively. This is called complementary base pairing. In RNA, the complement of adenine is uracil (U) instead of thymine.
Other notable purines are hypoxanthine (4), xanthine (5), theobromine (6), caffeine (7), uric acid (8) and isoguanine (9).
Functions
Aside from DNA and RNA, purines are biochemically significant components in a number of other important biomolecules, such as ATP, GTP, cyclic AMP, NADH, and coenzyme A. Purine (1) itself, has not been found in nature, but it can be produced by organic synthesis.
They may also function directly as neurotransmitters, acting upon purinergic receptors. Adenosine activates adenosine receptors.
History
The name 'purine' (purum uricum) was coined by the German chemist Emil Fischer in 1884. He synthesized it for the first time in 1899.[2] The starting material for the reaction sequence was uric acid (8), which had been isolated from kidney stones by Scheele in 1776.[3] Uric acid (8) was reacted with PCl5 to give 2,6,8-trichloropurine (10), which was converted with HI and PH4I to give 2,6-diiodopurine (11). The product was reduced to purine (1) using zinc-dust.
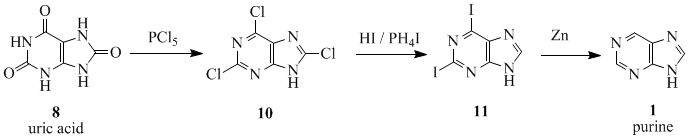
Metabolism
Many organisms have metabolic pathways to synthesize and break down purines.
Purines are biologically synthesized as nucleosides (bases attached to ribose).
Food sources
Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. Plant based diets are generally low in purines [1].
Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, and gravy.
A moderate amount of purine is also contained in beef, pork, poultry, fish and seafood, asparagus, cauliflower, spinach, mushrooms, green peas, lentils, dried peas, beans, oatmeal, wheat bran, wheat germ, and hawthorn.[4]
Higher levels of meat and seafood consumption are associated with an increased risk of gout, whereas a higher level of consumption of dairy products is associated with a decreased risk. Moderate intake of purine-rich vegetables or protein is not associated with an increased risk of gout.[5]
Laboratory synthesis
In addition to in vivo synthesis of purines in purine metabolism, purine can also be created artificially.
Purine (1) is obtained in good yield when formamide is heated in an open vessel at 170 oC for 28 hours.[6]
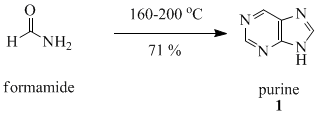
This reaction and others like it have been discussed in the context of the origin of life.[7]
Procedure:[6] Formamide (45 grams) was heated in an open vessel with a condenser for 28 hours in an oil bath at 170-190 oC. After removing excess formamide (32.1 grams) by vacuum distillation, the residue was refluxed with methanol. The methanol solvent was filtered, the solvent removed from the filtrate by vacuum distillation, and almost pure purine obtained; yield 4.93 grams (71% yield from formamide consumed). Crystallization from acetone afforded purine as colorless crystals; melting point 218 oC.
Oro, Orgel and co-workers have shown that four molecules of HCN tetramerize to form diaminomaleodinitrile (12), which can be converted into almost all natural-occurring purines.[8][9][10][11][12]

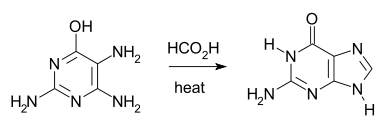
The Traube purine synthesis (1900) is a classic reaction (named after Wilhelm Traube) between an amine-substituted pyrimidine and formic acid.[13]
See also
- Simple aromatic rings
- Pyrimidine
References
- ↑ Rosemeyer, H. Chemistry & Biodiversity 2004, 1, 361.
- ↑ Fischer, E. Berichte der Deutschen Chemischen Gesellschaft 1899, 32, 2550.
- ↑ Scheele, V. Q. Examen Chemicum Calculi Urinari, Opuscula, 1776, 2, 73.
- ↑ Gout Diet: Limit High Purine Foods
- ↑ NEJM - Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men
- ↑ 6.0 6.1 Yamada, H.; Okamoto, T. (1972). "A One-step Synthesis of Purine Ring from Formamide". Chemical & Pharmaceutical Bulletin 20: 623. http://www.journalarchive.jst.go.jp/english/jnlabstract_en.php?cdjournal=cpb1958&cdvol=20&noissue=3&startpage=623.
- ↑ Saladino et al.; Crestini, Claudia; Ciciriello, Fabiana; Costanzo, Giovanna; Mauro, Ernesto (2006). "About a Formamide-Based Origin of Informational Polymers: Syntheses of Nucleobases and Favourable Thermodynamic Niches for Early Polymers". Origins of Life and Evolution of Biospheres 36 (5-6): 523–531. doi:10.1007/s11084-006-9053-2. PMID 17136429.
- ↑ Sanchez, R. A.; Ferris, J. P.; Orgel, L. E. Journal of Molecular Biology, 1967, 30, 223.
- ↑ Ferris, J. P.; Orgel, L. E. Journal of the American Chemical Society, 1966, 88, 1074.
- ↑ Ferris, J. P.; Kuder, J. E.; Catalano, O. W. Science, 1969, 166, 765.
- ↑ Oro, J.; Kamat, J. S. Nature, 1961, 190, 442.
- ↑ Houben-Weyl, Vol . E5, p. 1547
- ↑ Organic Syntheses Based on Name Reactions, Alfred Hassner, C. Stumer ISBN 008043259X 2002
External links
|
|||||||||||||||||||||||||||