Thymine
| Thymine | |
|---|---|
 |
|
 |
 |
|
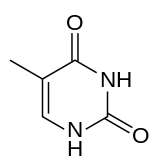
5-Methylpyrimidine-2,4(1H,3H)-dione
|
|
|
Other names
5-methyluracil
|
|
| Identifiers | |
| CAS number | 65-71-4 |
| MeSH | Thymine |
|
SMILES
CC1=CNC(=O)NC1=O
|
|
| Properties | |
| Molecular formula | C5H6N2O2 |
| Molar mass | 126.11334 g/mol |
| Melting point |
316–317 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Thymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine(T) binds to adenine (A) via two hydrogen bonds to assist in stabilizing the nucleic acid structures.
Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with one, two, or three phosphoric acid groups, creating, respectively, TMP, TDP, or TTP (thymidine mono-, di-, or triphosphate).
One of the common mutations of DNA involves two adjacent thymines or cytosine, which, in presence of ultraviolet light, may form thymine dimers, causing "kinks" in the DNA molecule that inhibit normal function.
Thymine could also be a target for actions of 5-fluorouracil (5-FU) in cancer treatment. 5-FU can be a metabolic analog of thymine (in DNA synthesis) or uracil (in RNA synthesis). Substitution of this analog inhibits DNA synthesis in actively-dividing cells.
Thymine bases are frequently oxidized to hydantoins over time after the death of an organism.[1]
See also
|
References
- ↑ Hofreiter M., Serre D., Poinar H.N., Kuch M., and Paabo S. Nature Reviews Genetics (2001) 2:353.
External links
|
|||||||||||||||||||||||||||