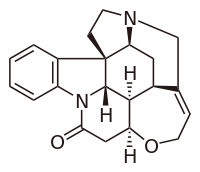
Strychnine
| Strychnine | |
|---|---|
 |
|
 |
|
|
Other names
Strychnidin-10-one
|
|
| Identifiers | |
| CAS number | 57-24-9 |
| PubChem | 441071 |
|
SMILES
[H][C@]([C@@](C(C=CC=C7)
=C7N34)5[C@H]6N(CC5)C2)3 [C@@]1([H])[C@@H](C6)[C@] 2=CCO[C@H]1CC4=O |
|
| Properties | |
| Molecular formula | C21H22N2O2 |
| Molar mass | 334.41 |
| Melting point | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Strychnine (pronounced /ˈstrɪkniːn/; also US: /ˈstrɪknaɪn/ or /ˈstrɪknɪn/) is a very toxic (LD50 = c. 10 mg/kg), colorless crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine causes muscular convulsions and eventually death through asphyxia or sheer exhaustion. The most common source is from the seeds of the Strychnos nux vomica tree. Strychnine is one of the most bitter substances known. Its taste is detectable in concentrations as low as 1 ppm.
Contents |
Pharmacology
Strychnine acts as a blocker or antagonist at the inhibitory or strychnine-sensitive glycine receptor (GlyR), a ligand-gated chloride channel in the spinal cord and the brain.[1]
Although it is best known as a poison, small doses of strychnine were once used in medications as a stimulant, as a laxative, and as a treatment for other stomach ailments. A 1934 drug guide for nurses described it as "among the most valuable and widely prescribed drugs".[2] Strychnine's stimulant effects also led to its use historically for enhancing performance in sports.[3] Because of its high toxicity and tendency to cause convulsions, the use of strychnine in medicine was eventually abandoned once safer alternatives became available.
The dosage for medical use was cited as between "1/60th grain–1/10th grain", which is between 1.1 milligrams and 6.4 milligrams in metric measures. In normal circumstances, the maximum dosage used was 3.2 mg, half of a "full dose".[4] A lethal dose was cited as 1/2 a grain (32 mg)
History
Strychnine was first isolated from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Its structure was determined by Sir Robert Robinson and Herman Leuchs. In 1952, Robinson wrote that "For its molecular size it is the most complex substance known."[5]
The first total synthesis of this molecule was reported by R. B. Woodward and his coworkers in 1954 (see: strychnine total synthesis).[6] X-ray crystallography studies confirmed the natural configuration of the molecule in 1956.[5] In 1963, a complete paper was published by Woodward and his coworkers.[7][8] K. C. Nicolaou calls the published synthesis "a spectacular achievement of organic synthesis".[5] Other syntheses have been published.[9][10]
See also
- Avicide
- Strychnine poisoning
- The Mysterious Affair at Styles
- "Poisoning Pigeons in the Park"
- Wieland-Gumlich aldehyde
- Denatonium
References
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 137–8. ISBN 978-0-87893-697-7.
- ↑ Principal Drugs and Their Uses, A.L. Morton, Faber and Faber, London, 1934
- ↑ Performance-Enhancing Substances in Sport and Exercise, Michael S. Bahrke and Charles Yesalis, Human Kinetics, 2002, ISBN 0736036792 Google Books
- ↑ "Nux Vomica. | Henriette's Herbal Homepage". Henriettesherbal.com. http://www.henriettesherbal.com/eclectic/potter-comp/strychnos-nux.html. Retrieved 2010-01-31.
- ↑ 5.0 5.1 5.2 Nicolaou, K. C.; E. J. Sorensen (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. pp. 21, 40. ISBN 3-527-29284-5.
- ↑ R. B. Woodward, Michael P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker (1954). "The total synthesis of strychnine". J. Am. Chem. Soc. 76: 4749. doi:10.1021/ja01647a088.
- ↑ R. B. Woodward, Michael P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker (1963). "The total synthesis of strychnine". Tetrahedron 19: 247.
- ↑ "Woodward Synthesis of Strychnine". http://www.chem.wisc.edu/areas/reich/syntheses/Strychnine-Woodward-syn.htm.
- ↑ "Overman Synthesis of Strychnine". http://www.chem.wisc.edu/areas/reich/syntheses/Strychnine-Overman-syn.htm.
- ↑ "Shibasaki Synthesis of Strychnine". http://www.chem.wisc.edu/areas/reich/syntheses/Strychnine-Shibasaki-syn.htm.
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||