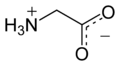
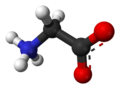
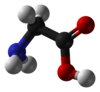
Glycine
| Glycine[1] | |
|---|---|
 |
 |
 |
 |
|
Glycine
|
|
|
Other names
Aminoethanoic acid
Aminoacetic acid |
|
| Identifiers | |
| Abbreviations | Gly, G |
| CAS number | 56-40-6 |
| PubChem | 750 |
| ChemSpider | 730 |
| EC-number | 200-272-2 |
|
SMILES
NCC(O)=O
|
|
| Properties | |
| Molecular formula | C2H5NO2 |
| Molar mass | 75.07 g mol−1 |
| Appearance | white solid |
| Density | 1.1607 g/cm3 |
| Melting point |
233 °C (decomposition) |
| Solubility in water | 25 g/100 mL |
| Solubility | soluble in ethanol, pyridine insoluble in ether |
| Acidity (pKa) | 2.35 and 9.78 |
| Hazards | |
| LD50 | 2600 mg/kg (mouse, oral) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Glycine (abbreviated as Gly or G)[2] is an organic compound with the formula NH2CH2COOH. With only a hydrogen atom as its side chain, glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG.
Glycine is a colourless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is not chiral. It can fit into hydrophilic or hydrophobic environments, due to its single hydrogen atom side chain.
Contents |
Production and key properties
Glycine was discovered in 1820, by Henri Braconnot who boiled gelatin with sulfuric acid.[3]
Glycine is manufactured industrially by treating chloroacetic acid with ammonia:[4]
- ClCH2COOH + 2 NH3 → H2NCH2COOH + NH4Cl
About 15M kg are produced annually in this way.[5]
In the USA (GEO Specialty Chemicals, Inc.) and in Japan by Shoadenko, glycine is produced via the Strecker amino acid synthesis.[6]
There are two producers of glycine in the United States. Chattem Chemicals, Inc., purchased by Sun Pharmaceutical, who is an international pharmaceutical company based in Mumbai, India and GEO Specialty Chemicals, Inc., who purchased the glycine and naphthalene sulfonate production facilities of Dow/Hampshire Chemical Corp.[6][7]
Chattem's manufacturing process ("MCA" process) occurs in batches and results in a finished product with some residual chloride but no sulfate, while GEO’s manufacturing process is considered a semi-batch process and results in a finished product with some residual sulfate but no chloride.
Its pK values are 2.35 and 9.78, so above pH 9.78, most of the glycine exists as the anionic amine, H2NCH2CO2-. Below pH 2.35, its solutions contain mostly the cationic carboxylic acid H3N+CH2CO2H. Its isoelectric point (pI) is 6.06.
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate. In most organisms, the enzyme Serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[8]
- serine + tetrahydrofolate → glycine + N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:[8]
- CO2 + NH4+ + N5,N10-Methylene tetrahydrofolate + NADH + H+ → Glycine + tetrahydrofolate + NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35% glycine.[8]
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals involves the catalysis of glycine cleavage enzyme, the same enzyme also involved in the biosynthesis of glycine. The degradation pathway is the reverse of this synthetic pathway:[8]
- Glycine + tetrahydrofolate + NAD+ → CO2 + NH4+ + N5,N10-Methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.[8]
In the third pathway of glycine degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glycoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.[8]
Physiological function
The principal function of glycine is as a precursor to proteins. It is also a building block to numerous natural products.
As a biosynthetic intermediate
In higher eukaryotes, D-Aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA. Glycine provides the central C2N subunit of all purines.[8]
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg/kg in rats (oral),[9] and it usually causes death by hyperexcitability.
Commercial uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. Most glycine is manufactured as USP grade material for diverse uses. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine.
- Pharmaceutical grade glycine is produced for some pharmaceutical applications, such as intravenous injections, where the customer’s purity requirements often exceed the minimum required under the USP grade designation. Pharmaceutical grade glycine is often produced to proprietary specifications and is typically sold at a premium over USP grade glycine.
- Technical grade glycine, which may or may not meet USP grade standards, is sold for use in industrial applications; e.g., as an agent in metal complexing and finishing. Technical grade glycine is typically sold at a discount to USP grade glycine.[10]
Animal and human foods
Other markets for USP grade glycine include its use an additive in pet food and animal feed. For humans, glycine is sold as a sweetener/taste enhancer. Food supplements and protein drinks contain glycine. Certain drug formulations include glycine to improve gastric absorption of the drug.
Cosmetics and miscellaneous applications
Glycine serves as a buffering agent in antacids, analgesics, antiperspirants, cosmetics, and toiletries.
Many miscellaneous products use glycine or its derivatives, such as the production of rubber sponge products, fertilizers, metal complexants.[11]

Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicide Glyphosate. Glyphosate (N-(phosphonomethyl) glycine) is a non-selective systemic herbicide used to kill weeds, especially perennials and broadcast or used in the cut-stump treatment as a forestry herbicide. Initially, Glyphosate was sold only by Monsanto under the Monsanto tradename Roundup, but is no longer under patent.
Presence in space
The detection of glycine in the interstellar medium has been debated.[12] In 2008, the glycine-like molecule amino acetonitrile was discovered in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius by the Max Planck Institute for Radio Astronomy.[13] In 2009, glycine sampled in 2004 from comet Wild 2 by the NASA spacecraft Stardust was confirmed, the first discovery of extraterrestrial glycine. That mission's results bolstered the theory of panspermia, which claims that the "seeds" of life are widespread throughout the universe.[14]
References
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 4386.
- ↑ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer & F.G. Hopkins. ed. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co.. p. 82. http://books.google.com/books?id=7JM8AAAAIAAJ&ots=orkEuT2SrL&pg=PA112. Retrieved January 18, 2010.
- ↑ Ingersoll, A. W.; Babcock, S. H. (1932), "Hippuric acid", Org. Synth. 12: 40, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0328; Coll. Vol. 2: 328.
- ↑ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker “Amino Acids” in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_057.pub2
- ↑ 6.0 6.1 http://www.usitc.gov/trade_remedy/731_ad_701_cvd/investigations/2007/glycine_from_india_japan_korea/preliminary/DOC/Glycine%20Conference%20(prelim).wpd
- ↑ U.S. International Trade Commission, "Glycine From China." Investigation No. 731-TA-718 (Second Review), Publication No. 3810, October 2005
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, pp. 127, 675–77, 844, 854, ISBN 0-7167-4339-6.
- ↑ "Safety (MSDS) data for glycine". The Physical and Theoretical Chemistry Laboratory Oxford University. 2005. http://physchem.ox.ac.uk/MSDS/GL/glycine.html. Retrieved 2006-11-01.
- ↑ http://hotdocs.usitc.gov/docs/pubs/701_731/pub3921.pdf
- ↑ "Notice of Preliminary Determination of Sales at Less Than Fair Value: Glycine From India" Federal Register 72 (7 November 2007): 62827.
- ↑ Snyder LE, Lovas FJ, Hollis JM, et al. (2005). "A rigorous attempt to verify interstellar glycine". Astrophys J 619 (2): 914–930. doi:10.1086/426677.
- ↑ Staff. "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius 27 March 2008 - Science Daily". http://www.sciencedaily.com/releases/2008/03/080326161658.htm. Retrieved 2008-09-16.
- ↑ Reuters. "Building block of life found on comet - Thomson Reuters 2009". http://www.reuters.com/article/scienceNews/idUSTRE57H02I20090818. Retrieved 2009-08-18.
Further reading
On attempts to detect glycine in interstellar medium
- Kuan YJ, Charnley SB, Huang HC, et al. (2003). "Interstellar glycine". Astrophys J 593 (2): 848–867. doi:10.1086/375637.
- Rachel Nowak. "Amino acid found in deep space - 18 July 2002 - New Scientist". http://www.newscientist.com/news/news.jsp?id=ns99992558. Retrieved 2007-07-01.
External links
- Glycine at PDRHealth.com
- Glycine cleavage system
- Glycine Therapy - A New Direction for Schizophrenia Treatment?
- "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius". ScienceDaily. 27 March 2008. http://www.sciencedaily.com/releases/2008/03/080326161658.htm.
- Guochuan E. Tsai (1 December 2008). "A New Class of Antipsychotic Drugs: Enhancing Neurotransmission Mediated by NMDA Receptors". Psychiatric Times 25 (14). http://www.psychiatrictimes.com/display/article/10168/1357569.
- ChemSub Online (Glycine).
- NASA scientists have discovered glycine, a fundamental building block of life, in samples of comet Wild 2 returned by NASA's Stardust spacecraft.
|
||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||