Etanercept
 |
|
|---|---|
| Identifiers | |
| CAS number | 185243-69-0 |
| ATC code | L04AB01 |
| PubChem | SID 10099 |
| DrugBank | DB00005 |
| Chemical data | |
| Formula | C2224H3475N621O698S36 |
| Mol. mass | 51234.9 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 58–76% (SC) |
| Metabolism | Reticuloendothelial system (speculative) |
| Half-life | 70–132 hours |
| Therapeutic considerations | |
| Pregnancy cat. | B2 (Au), B (U.S.) |
| Legal status | S4 (Au), POM (UK), ℞-only (U.S.) |
| Routes | Subcutaneous |
Etanercept (trade name Enbrel) is a drug that treats autoimmune diseases by interfering with the tumor necrosis factor (TNF, a part of the immune system) by acting as a TNF inhibitor.
Etanercept is a fusion protein produced through expression of recombinant DNA. That is, it is a product of a DNA "construct" engineered to link the human gene for soluble TNF receptor 2 to the gene for the Fc component of human immunoglobulin G1 (IgG1). Expression of the construct produces a continuous protein "fusing" TNF receptor 2 to IgG1. Production of Etanercept is accomplished by the large-scale culturing of cells that have been "cloned" to express this recombinant DNA construct.
The prototypic fusion protein was first synthesized and shown to be highly active and unusually stable as a modality for blockade of TNF in vivo in the early 1990s by Bruce A. Beutler, an academic researcher then at the University of Texas Southwestern Medical Center at Dallas, and his colleagues.[1][2][3] These investigators also patented the protein,[4] selling all rights to its use to Immunex, a biotechnology company that was acquired by Amgen in 2002.[5]
It is a large molecule, with a molecular weight of 150 kDa., that binds to TNFα and decreases its role in disorders involving excess inflammation in humans and other animals, including autoimmune diseases such as ankylosing spondylitis,[6] juvenile rheumatoid arthritis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and, potentially, in a variety of other disorders mediated by excess TNFα.
This therapeutic potential is based on the fact that TNF-alpha is the "master regulator" of the inflammatory response in many organ systems.[7]
In North America, etanercept is co-marketed by Amgen and Pfizer under the trade name Enbrel in two separate formulations, one in powder form, the other as a pre-mixed liquid. Wyeth is the sole marketer of Enbrel outside of North America excluding Japan where Takeda Pharmaceuticals markets the drug.
Etanercept is an example of a protein-based drug created using the tools of biotechnology and conceived through an understanding afforded by modern cell biology.
Contents |
Development
Etanercept was developed by researchers at Immunex, and was released for commercial use in late 1998, soon after the release of infliximab (Remicade) – the first chimeric monoclonal antibody against TNFα to be marketed for clinical use.
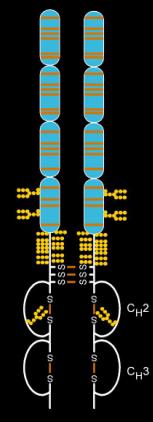
Etanercept is a dimeric molecule,[8] and this dimeric structure is necessary for its proper therapeutic activity. During its development at Immunex Corporation an earlier monomeric version did not have sufficient biologic activity.
Mode of action
It reduces the effect of naturally present TNF, and hence is a TNF inhibitor, functioning as a decoy receptor that binds to TNF.[9]
Tumor necrosis factor-alpha (TNFα) is a cytokine produced by monocytes and macrophages, two types of white blood cells. It mediates the immune response by increasing the transport of white blood cells to sites of inflammation, and through additional molecular mechanisms which initiate and amplify inflammation. Inhibition of its action by etanercept reduces the inflammatory response which is especially useful for treating autoimmune diseases.
There are two types of TNF receptors: those found embedded in white blood cells that respond to TNF by releasing other cytokines, and soluble TNF receptors which are used to deactivate TNF and blunt the immune response. In addition, TNF receptors are found on the surface of virtually all nucleated cells (red blood cells, which are not nucleated, do not contain TNF receptors on their surface). Etanercept mimics the inhibitory effects of naturally occurring soluble TNF receptors, the difference being that etanercept, because it is a fusion protein rather than a simple TNF receptor, has a greatly extended half-life in the bloodstream, and therefore a more profound and long-lasting biologic effect than a naturally occurring soluble TNF receptor.[10]
Structure
Etanercept is made from the combination of two naturally occurring soluble human 75-kilodalton TNF receptors linked to an Fc portion of an IgG1. The effect is an artificially engineered dimeric fusion protein.
Licensed indications
In the USA the FDA has licenced Enbrel for :
- Moderate to Severe Rheumatoid Arthritis (RA) (Nov 1998)
- Moderate to Severe Polyarticular Juvenile Idiopathic Arthritis (JIA) (1999)
- Psoriatic Arthritis (2002)
- Ankylosing Spondylitis (AS) (July 2003)[11]
- Moderate to Severe Plaque Psoriasis (April 2004)
Administration
Enbrel is marketed as a lyophylized powder in 25 mg vials which must be reconstituted with a diluent and then injected subcutaneously, typically by the patient at home.
Because patients with arthritis found the reconstitution procedure difficult, it was made available as pre-filled 50 mg/ml syringes in late 2004 and a single-use 50 mg autoinjector "pen" was brought to market in mid-2006.[12]
It cannot be administered orally, because the digestive system would destroy the drug.
FDA approved dose is 25 mg BIW (twice weekly) or 50 mg QW (once weekly).
Safety
All TNF inhibitors are immunosuppressants. After a number of studies and reports of adverse reactions in patients receiving anti-TNF alpha therapy (including serious and sometimes fatal blood disorders, infections, rare reports of lymphoma and solid tissue cancers, rare reports of serious liver injury, and rare reports of demyelinating central nervous system disorders), rare reports of congestive heart failure, the U.S. Food and Drug Administration issued a warning to doctors appearing in the respective product labeling of these drugs instructing them to screen and monitor potential patients more carefully.[13]
Although these three agents - etanercept, infliximab and adalimumab - are all biologic anti-TNF therapeutics, their methods of administration, dosing, and side effect profiles are somewhat different. These differences may be accounted for by fundamental differences in their biologic structure. Both infliximab and adalimumab fix complement, and have the ability to lyse cells. While potentially contributing to their therapeutic efficacy in disorders such as Crohn's disease (for which both of these monoclonal antibodies are now FDA-approved), these mABs also carry black-box warnings. In addition infliximab has a higher propensity for the development of anaphylaxis, perhaps as a result both of its chimeric structure and its intravenous route of administration.
On May 2, 2008, the FDA placed a black box warning on etanercept due to a number of serious infections associated with the drug.[14]
Experimental and off-label uses
Given the central role of TNF-alpha in many diseases, etanercept is being studied as treatment for a number of these disease, including over 150 clinical trials.[15] This includes certain forms of vasculitis (such as Wegener's granulomatosis, in which it was not effective).[16]
Alzheimer's disease: A 2006 pilot study showed small but significant improvements in various cognitive rating scales in patients with Alzheimer's disease (AD) after treatment with etanercept.[17] A further study, administering to a single AD patient via perispinal infusion, showed rapid and significant improvement in Alzheimer's symptoms.[18] A small number of US physicians offer etanercept treatment for AD at a cost of $10,000 to $40,000 per annum.[19]
Generic etanercept
While the patent on Enbrel expires on October 23 2012[20] in the United States, it is unlikely that a generic will be available at that time. As a biologic, etanercept is subject to different laws than chemical formulations. Currently many countries, including the United States, do not permit the manufacture of generic biologics. However, the European Union does currently have in place a system to approve generic biologics (biosimilars) which "requires mandatory clinical testing and periodic review"[21]. The United States Congress is currently reviewing a bill to introduce such a process in the United States; however, the bill has been stalled in the House Judiciary Committee for 14 months as of June 2010[22].
Similar agents
- Soluble TNF receptor
- Pegsunercept
- Anti-TNF monoclonal antibodies
- Infliximab
- Adalimumab
- Certolizumab pegol
References
- ↑ Peppel,K. et al. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J.Exp.Med. 174(6):1483-9, 1991
- ↑ Peppel,K. et al. Expression of a TNF inhibitor in transgenic mice. J.Immunol. 151(10):5699-703, 1993
- ↑ Kolls,J. et al. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc.Natl.Acad.Sci.USA 91(1):215-9, 1994
- ↑ U.S. Patent number: 5,447,851
- ↑ "Arthritis Drug Effective for Depression in Psoriasis Sufferers". http://www.dukemednews.org/news/article.php?id=9419. Retrieved 2008-01-10.
- ↑ Braun J, McHugh N, Singh A, Wajdula JS, Sato R (2007). "Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly". Rheumatology (Oxford) 46 (6): 999–1004. doi:10.1093/rheumatology/kem069. PMID 17389658. http://rheumatology.oxfordjournals.org/cgi/content/full/46/6/999.
- ↑ "TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases - Nature Medicine". http://www.nature.com/nm/journal/v9/n10/full/nm939.html. Retrieved 2008-01-10.
- ↑ Smith KJ, Skelton HG (2001). "Rapid onset of cutaneous squamous cell carcinoma in patients with rheumatoid arthritis after starting tumor necrosis factor alpha receptor IgG1-Fc fusion complex therapy". J. Am. Acad. Dermatol. 45 (6): 953–6. doi:10.1067/mjd.2001.117725. PMID 11712048. http://linkinghub.elsevier.com/retrieve/pii/S0190-9622(01)47789-9.
- ↑ Zalevsky J, Secher T, Ezhevsky SA, et al. (August 2007). "Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection". J. Immunol. 179 (3): 1872–83. PMID 17641054.
- ↑ Madhusudan S, Muthuramalingam SR, Braybrooke JP, et al. (2005). "Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer". J. Clin. Oncol. 23 (25): 5950–9. doi:10.1200/JCO.2005.04.127. PMID 16135466. http://jco.ascopubs.org/cgi/content/full/23/25/5950.
- ↑ http://www.fda.gov/bbs/topics/ANSWERS/2003/ANS01240.html FDA
- ↑ "Enbrel and Humira Now Have Automated Delivery System". http://www.hopkins-arthritis.org/arthritis-news/2006/automated_delivery.html. Retrieved 2008-01-10.
- ↑ "Prescribing Information - ENBREL". http://www.enbrel.com/prescribing-information.jsp. Retrieved 2008-01-10.
- ↑ "Wyeth and Amgen heighten warning of life-threatening infections on skin drug Enbrel". http://money.cnn.com/news/newsfeeds/articles/apwire/21965cf0a650a8b5897142bfcbccb427.htm. Retrieved 2008-05-02.
- ↑ FDA Clinical Trials database
- ↑ Wegener's Granulomatosis Etanercept Trial (WGET) Research Group (2005). "Etanercept plus standard therapy for Wegener's granulomatosis". N. Engl. J. Med. 352 (4): 351–61. doi:10.1056/NEJMoa041884. PMID 15673801. http://content.nejm.org/cgi/content/full/352/4/351.
- ↑ Tobinick Edward L., Gross H, Weinberger A, Cohen H (2006). "TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study". MedGenMed 8 (2): 25. PMID 16926764. PMC 1785182. http://www.medscape.com/viewarticle/529176.
- ↑ Tobinick Edward L., Gross H. (2008). "Rapid cognitive improvement in Alzheimer's disease following perispinal etanercept administration". J. Neuroinflammation 5 (2): 2. doi:10.1186/1742-2094-5-2. PMID 18184433. PMC 2211476. http://www.jneuroinflammation.com/content/5/1/2.
- ↑ http://www.newscientist.com/channel/health/mental-health/mg19926681.600-is-miracle-alzheimers-cure-too-good-to-be-true.html
- ↑ http://www.uspto.gov/patents/resources/terms/156.html
- ↑ http://www.law.duke.edu/journals/dltr/articles/2008dltr0009.html
- ↑ http://www.govtrack.us/congress/bill.xpd?bill=h111-1427
External links
- Amgen/Wyeth Enbrel site
- A case study on the development of Enbrel until Immunex's acquisition by Amgen
|
|||||||||||||||||||||||||||||||||||||||||||||||||||