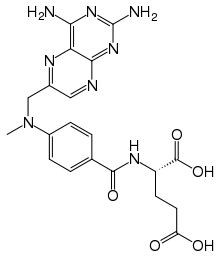
Methotrexate
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| (2S)-2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid | |
| Identifiers | |
| CAS number | 59-05-2 |
| ATC code | L01BA01 L04AX03 |
| PubChem | CID 126941 |
| DrugBank | APRD00353 |
| ChemSpider | 112728 |
| Chemical data | |
| Formula | C20H22N8O5 |
| Mol. mass | 454.44 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 17–90% |
| Metabolism | hepatic |
| Half-life | 3–15 hours (dose dependent) |
| Excretion | renal 48–100% |
| Therapeutic considerations | |
| Pregnancy cat. | D(AU) X(US) |
| Legal status | Prescription Only (S4) (AU) POM (UK) ℞-only (US) |
| Routes | oral, IV, IM, SC, intrathecal |
| |
|
Methotrexate (rINN) (pronounced /mɛθɵˈtrɛkseɪt/), abbreviated MTX and formerly known as amethopterin, is an antimetabolite and antifolate drug used in treatment of cancer, autoimmune diseases and as an abortifacient in the induction of medical abortions. It acts by inhibiting the metabolism of folic acid. Methotrexate began to replace the more powerful and toxic antifolate aminopterin starting in the 1950s, and the two should not be confused. The drug was developed by Yellapragada Subbarao.
History
In 1947, a team of researchers led by Sidney Farber showed that aminopterin, a chemical analogue of folic acid developed by Yellapragada Subbarao Lederle, could induce remission in children with acute lymphoblastic leukemia. The development of folic acid analogues had been prompted by the discovery that the administration of folic acid worsened leukemia, and that a diet deficient in folic acid could, conversely, produce improvement; the mechanism of action behind these effects was still unknown at the time.[1] Other analogues of folic acid were in development, and by 1950, methotrexate (then known as amethopterin) was being proposed as a treatment for leukemia.[2] Animal studies published in 1956 showed that the therapeutic index of methotrexate was better than that of aminopterin, and clinical use of aminopterin was thus abandoned in favor of methotrexate. In that same year, methotrexate was found to be a curative treatment for choriocarcinoma—a solid tumor, unlike leukemia, which is a cancer of the blood.[1] The drug was then investigated as a treatment for many other cancers, alone or in combination with other drugs, and was studied for other, non-cancer indications in the 1970s. In 1988, it was approved by the U.S. Food and Drug Administration (FDA) to treat rheumatoid arthritis.[3]
Mode of action
Methotrexate competitively inhibits dihydrofolate reductase (DHFR), an enzyme that participates in the tetrahydrofolate synthesis.[4] The affinity of methotrexate for DHFR is about one thousand-fold that of folate for DHFR. Dihydrofolate reductase catalyses the conversion of dihydrofolate to the active tetrahydrofolate. Folic acid is needed for the de novo synthesis of the nucleoside thymidine, required for DNA synthesis. Also, folate is needed for purine base synthesis, so all purine synthesis will be inhibited. Methotrexate, therefore, inhibits the synthesis of DNA, RNA, thymidylates, and proteins.
Methotrexate acts specifically during DNA and RNA synthesis, and thus it is cytotoxic during the S-phase of the cell cycle. Logically, it therefore has a greater toxic effect on rapidly dividing cells (such as malignant and myeloid cells, and GI & oral mucosa), which replicate their DNA more frequently, and thus inhibits the growth and proliferation of these non-cancerous cells as well as causing the side effects listed below.
Lower doses of methotrexate have been shown to be very effective for the management of rheumatoid arthritis, Crohn's disease, and psoriasis. For the treatment of rheumatoid arthritis, patients should supplement their diet with folate. In these cases inhibition of dihydrofolate reductase (DHFR) is not thought to be the main mechanism, but rather the inhibition of enzymes involved in purine metabolism, leading to accumulation of adenosine, or the inhibition of T cell activation and suppression of intercellular adhesion molecule expression by T cells.[5]
Uses
In cancer chemotherapy
Methotrexate was originally used as part of combination chemotherapy regimens to treat many kinds of cancers. It is still the mainstay for the treatment of many neoplastic disorders including acute lymphoblastic leukemia.
Medical termination of pregnancy
Methotrexate is commonly used (generally in combination with misoprostol) to terminate pregnancies during the early stages (i.e. as an abortifacient). It is also used to treat ectopic pregnancies.[6] In the case of early missed miscarriage (particularly a blighted ovum), in which fetal demise has occurred but the body has not expelled the fetus, methotrexate may be used to help the body begin the miscarriage process.
Other uses
It has come into use as a treatment for some autoimmune diseases, including Myasthenia Gravis, polymyositis, dermatomyositis, inclusion body myositis, ankylosing spondylitis, Crohn's disease, psoriasis, pustular psoriasis, psoriatic arthritis, rheumatoid arthritis, Wegener's granulomatosis, Adult-Onset Still's Disease, and scleroderma (see disease-modifying antirheumatic drugs). A parallel use with TNFα blockers, such as adalimumab, infliximab, or etanercept, has been shown to markedly improve symptoms.[7]
It is also sometimes used to treat a rare condition called Behçet's disease where it is taken weekly, along with folic acid daily. In the case of immune disorders, such as Behçet's disease and rheumatoid disorders, it is believed that the clinical goal of the low dose methotrexate regimen is to inhibit AICAR transformylase, which leads to increased AICA ribose (AICAR transformylase's substrate). The AICA ribose inhibits adenosine deaminase, resulting in a build-up of extracellular adenosine. Extracellular adenosine inhibits the expression of IL-2 receptors on circulating T-lymphocytes, causing a suppression of the immune system, and thus ameliorating the effects of the immune disorder.
Pharmacokinetics
Methotrexate is a weak dicarboxylic acid with pKa 4.8 and 5.5, and thus it is mostly ionized at physiologic pH. Oral absorption is saturatable and thus dose-dependent, with doses less than 40 mg/m2 having 42% bioavailability and doses greater than 40 mg/m2 only 18%. Mean oral bioavailability is 33% (13-76% range), and there is no clear benefit to subdividing an oral dose. Mean intramuscular bioavailability is 76%.
Methotrexate is metabolized by intestinal bacteria to the inactive metabolite 4-amino-4-deoxy-N-methylpteroic acid (DAMPA) and accounts for less than 5% loss of the oral dose.
Factors that decrease absorption include food, oral non-absorbable antibiotics (e.g. vancomycin, neomycin, and bacitracin), and more rapid transit through the gastrointestinal tract (GI) tract such as diarrhea, while slower transit time in the GI tract from constipation will increase absorption. Methotrexate is also administered in the placenta accreta, inhibiting the blood circulation to the target site.
Drug Interactions
There is a risk of a severe adverse reactions if penicillins or related antibiotics are used alongside methotrexate. There have been numerous case reports of possible decreased urinary excretion of methotrexate due to competition by some acidic drugs like beta-lactams (penicillins, cephalosporins, carbapenems, and monobactams) for secretion in the renal tubule, with toxicity resulting due to increased blood methotrexate concentration.
Administration
It can be taken orally or administered by injection (subcutaneous, intramuscular, intravenous or intrathecal). Although daily preparations are occasionally used, most patients take weekly doses, which decreases the risk of certain side-effects. (People taking this medicine must get appropriate tests done every 6 weeks in hospital, especially older people, to make sure no potentially fatal damage is being done to blood cells and immune system)
Adverse effects
Possible side effects can include anemia, neutropenia, increased risk of bruising, hair loss, nausea and vomiting, dermatitis and diarrhea. A small percentage of patients develop hepatitis, and there is an increased risk of pulmonary fibrosis where dry cough can be an important sign.
The higher doses of methotrexate often used in cancer chemotherapy can cause toxic effects to the rapidly-dividing cells of bone marrow and gastrointestinal mucosa. The resulting myelosuppression and mucositis are often prevented (termed Leucovorin "rescue"- as this is the folic acid based drug used).
Methotrexate is a highly teratogenic drug and categorized in Pregnancy Category X by the FDA. Women must not take the drug during pregnancy, if there is a risk of becoming pregnant, or if they are breastfeeding. Men who are trying to get their partner pregnant must also not take the drug. To engage in any of these activities (after discontinuing the drug), women must wait until the end of a full ovulation cycle and men must wait three months.
Reports of central nervous system reactions to methotrexate especially when given via the intrathecal route which include myelopathies and leucoencephalopathies.
Generally, the more "non-specific" action a pharmacological substance has, the more possible side effects can be expected. Methotrexate has like all "cell toxic" substances a broad array of possible adverse effects. Care should always be taken to read the manufacturer's original instructions for the preparation in question.
Here is a more thorough list of potential side effects for Methotrexate:
Most frequent
Ulcerative stomatitis, leukopenia, nausea, abdominal distress.
Other frequent
Hair loss, malaise, undue fatigue, chills and fever, dizziness and lowered resistance to infection.
Other rarer reactions
(related to or attributed to Methotrexate) nodulosis, vasculitis, arthralgia/myalgia, loss of libido/impotence, diabetes, osteoporosis, osteonecrosis, sudden death, reversible lymphomas, tumor lysis syndrome, soft tissue necrosis, anaphylactoid reactions.
By organ system:
Alimentary system
Anorexia, nausea, vomiting, diarrhea; Gingivitis, pharyngitis, stomatitis, hematemesis, melena, gastrointestinal ulceration/bleeding, enteritis, pancreatitis.
Blood/lymphatic system
Anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, thrombocytopenia, lymphadenopathy and lymphoproliferative disorders. Hypogammaglobulinemia.
Cardiovascular system
Pericarditis, pericardial effusion, hypotension, thromboembolic events (cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus.
Central nervous system
Headaches, drowsiness, blurred vision, transient blindness, speech impairment including dysarthria and aphasia, hemiparesis, paresis and convulsions. Occasional reports of transient subtle cognitive dysfunction, mood alteration (depression), unusual cranial sensations, leukoencephalopathy, encephalopathy.
Hepatobiliary system
Hepatotoxicity, acute hepatitis, chronic fibrosis/cirrhosis, decrease in serum albumin, liver enzyme elevations.
Immune system (infections)
Fatal opportunistic infections (Pneumocystis carinii pneumonia, pneumonia, sepsis, nocardiosis, histoplasmosis, cryptococcosis, Herpes zoster, Herpes simplex hepatitis and disseminated Herpes simplex).
Musculoskeletal system
Stress fracture.
Ophthalmic
Conjunctivitis, serious visual changes (without known cause).
Respiratory system
Respiratory fibrosis, respiratory failure, interstitial pneumonitis, and chronic interstitial obstructive pulmonary disease. Dry cough possibly being a symptom of these aforementioned conditions..
Dermatologic
Acne, rashes (Erythematous rashes), pruritus, urticaria, photosensitivity, pigmentary changes, alopecia, ecchymosis, telangiectasia, furunculosis, erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson Syndrome, skin necrosis, skin ulceration and exfoliative dermatitis.
Urogenital system
Severe nephropathy or renal failure, azotemia, cystitis, hematuria; defective oogenesis or spermatogenesis, transient oligospermia, menstrual dysfunction, vaginal discharge, and gynecomastia; infertility, abortion, crystalluria,fetal defects.[8]
References
- ↑ 1.0 1.1 Bertino JR (2000). "Methotrexate: historical aspects". In Cronstein BN, Bertino JR. Methotrexate. Basel: Birkhäuser. ISBN 9783764359591. http://books.google.com/?id=VCAFHzHAotsC. Retrieved on November 21, 2009 through Google Book Search.
- ↑ Meyer LM, Miller FR, Rowen MJ, Bock G, Rutzky J (1950). "Treatment of acute leukemia with amethopterin (4-amino, 10-methyl pteroyl glutamic acid)". Acta Haematologica 4 (3): 157–67. PMID 14777272.
- ↑ http://arthritis.about.com/od/mtx/p/methotrexate.htm
- ↑ P. T. Ravi Rajagopalan, Zhiquan Zhang, Lynn McCourt, Mary Dwyer, Stephen J. Benkovic, and Gordon G. Hammes (2002). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". PNAS 99 (21): 13481-13486. doi:10.1073/pnas.172501499. http://www.pnas.org/content/99/21/13481.full.
- ↑ Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H; (2005). "The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules". Clin Immunol. 114 (Feb.): 154–163. doi:10.1016/j.clim.2004.09.001. PMID 15639649.
- ↑ Mol F, Mol BW, Ankum WM, van der Veen F, Hajenius PJ (2008). "Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis". Hum. Reprod. Update 14 (4): 309–19. doi:10.1093/humupd/dmn012. PMID 18522946. http://humupd.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=18522946.
- ↑ Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, Martin Mola E, Pavelka K, Sany J, Settas L, Wajdula J, Pedersen R, Fatenejad S, Sanda M (2004). "Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial". Lancet 363 (9410): 675–81. doi:10.1016/S0140-6736(04)15640-7. PMID 15001324.
- ↑ http://www.drugs.com/sfx/methotrexate-side-effects.html
External links
- National Rheumatoid Arthritis Society (NRAS) article on Methotrexate
- Chembank entry on methotrexate
- Methotrexate general article from NIH
- Methotrexate Injection MedlinePlus article from NIH
- High Dose Methorexate as a potential treatment for Glioblastoma
- Patient Education - Methotrexate from American College of Rheumatology
- Methotrexate-resistant mutants of human dihydrofolate reductase for use as a selection marker in gene therapy as seen on Flintbox
- U.S. National Library of Medicine: Drug Information Portal - Methotrexate
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||