Glutathione
| Glutathione[1] | |
|---|---|
 |
|
 |
|
|
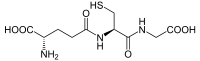
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid
|
|
|
Other names
γ-L-Glutamyl-L-cysteinylglycine
(2S)-2-Amino-5-[[(2R)-1-(carboxymethylamino)-1-oxo- 3-sulfanylpropan-2-yl]amino]-5-oxopentanoic acid |
|
| Identifiers | |
| Abbreviations | GSH |
| CAS number | 70-18-8 |
| PubChem | 124886 |
| ChemSpider | 111188 |
| MeSH | Glutathione |
|
SMILES
C(CC(=O)N[C@@H](CS)C(=O)NCC(=O)O)[C@@H](C(=O)O)N
|
|
| Properties | |
| Molecular formula | C10H17N3O6S |
| Molar mass | 307.32 g/mol |
| Melting point |
195 °C, 468 K, 383 °F |
| Solubility in water | Miscible |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Glutathione (GSH) is a tripeptide. It contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side chain. Glutathione, an antioxidant, helps protect cells from reactive oxygen species such as free radicals and peroxides.[2]
Thiol groups are reducing agents, existing at a concentration of approximately ~5 mM in animal cells. Glutathione reduces disulfide bond formed within cytoplasmic proteins to cysteines by serving as an electron donor. In the process, glutathione is converted to its oxidized form glutathione disulfide (GSSG). Glutathione is found almost exclusively in its reduced form, since the enzyme that reverts it from its oxidized form, glutathione reductase, is constitutively active and inducible upon oxidative stress. In fact, the ratio of reduced glutathione to oxidized glutathione within cells is often used scientifically as a measure of cellular toxicity.[3]
Contents |
Biosynthesis
Glutathione is not an essential nutrient since it can be synthesized from the amino acids L-cysteine, L-glutamic acid and glycine. The sulfhydryl (thiol) group (SH) of cysteine serves as a proton donor and is responsible for the biological activity of glutathione. Provision of this amino acid is the rate-limiting factor in glutathione synthesis by the cells since cysteine is relatively rare in foodstuffs. Furthermore, if released as the free amino acid, cysteine is toxic and spontaneously catabolized in the gastrointestinal tract and blood plasma.[4]
Glutathione is synthesized in two adenosine triphosphate-dependent steps:
- First, gamma-glutamylcysteine is synthesized from L-glutamate and cysteine via the enzyme gamma-glutamylcysteine synthetase (a.k.a. glutamate cysteine ligase, GCL). This reaction is the rate-limiting step in glutathione synthesis.
- Second, glycine is added to the C-terminal of gamma-glutamylcysteine via the enzyme glutathione synthetase.
Animal and insect glutamate cysteine ligase (GCL) is a heterodimeric enzyme composed of a catalytic (GCLC) and modulatory (GCLM) subunit. GCLC constitutes all the enzymatic activity, whereas GCLM increases the catalytic efficiency of GCLC. Mice lacking GCLC (i.e., all de novo GSH synthesis) die before birth.[5] Mice lacking GCLM demonstrate no outward phenotype, but exhibit marked decrease in GSH and increased sensitivity to toxic insults.[6][7][8]
While all cells in the human body are capable of synthesizing glutathione, liver glutathione synthesis has been shown to be essential. Following birth, mice with genetically-induced loss of GCLC (i.e., GSH synthesis) only in the liver die within 1 month of birth.[9]
The plant glutamate cysteine ligase (GCL) is a redox sensitive homodimeric enzyme, conserved in the plant kingdom.[10] In an oxidizing environment intermolecular disulfide bridges are formed and the enzyme switches to the dimeric active state. The mid-point potential of the critical cysteine paire is - 318 mV. In addition to the redox dependent control is the plant GCL enzyme feedback inhibited by GSH.[11] GCL is exclusively located in plastids and glutathione synthetase is dual-targeted to plastids and cytosol, thus are GSH and gamma-glutamylcysteine exported from the plastids.[12] Both glutathione biosynthesis enzymes are essential in plants, knock-outs of GCL and GS are embryo and seedling lethal.[13]
The biosynthesis pathway for glutathione is found in some bacteria, like cyanobacteria and proteobacteria, but is missing in many other bacteria. Most eukaryotes synthesize glutathione, including humans, but some do not, such as Leguminosae, Entamoeba, and Giardia. The only archaea that make glutathione are halobacteria.[14][15]
Function
Glutathione exists in reduced (GSH) and oxidized (GSSG) states. In the reduced state, the thiol group of cysteine is able to donate a reducing equivalent (H++ e-) to other unstable molecules, such as reactive oxygen species. In donating an electron, glutathione itself becomes reactive, but readily reacts with another reactive glutathione to form glutathione disulfide (GSSG). Such a reaction is possible due to the relatively high concentration of glutathione in cells (up to 5 mM in the liver). GSH can be regenerated from GSSG by the enzyme glutathione reductase.
In healthy cells and tissue, more than 90% of the total glutathione pool is in the reduced form (GSH) and less than 10% exists in the disulfide form (GSSG). An increased GSSG-to-GSH ratio is considered indicative of oxidative stress.
Glutathione has multiple functions:
- It is the major endogenous antioxidant produced by the cells, participating directly in the neutralization of free radicals and reactive oxygen compounds, as well as maintaining exogenous antioxidants such as vitamins C and E in their reduced (active) forms.>
- Regulation of the nitric oxide cycle[16], which is critical for life but can be problematic if unregulated[17]
- Through direct conjugation, it detoxifies many xenobiotics (foreign compounds) and carcinogens, both organic and inorganic. This includes heavy metals such as mercury, lead, and arsenic.
- It is essential for the immune system to exert its full potential, e.g. (1) modulating antigen presentation to lymphocytes, thereby influencing cytokine production and type of response (cellular or humoral) that develops, (2) enhancing proliferation of lymphocytes thereby increasing magnitude of response, (3) enhancing killing activity of cytotoxic T cells and NK cells, and (4) regulating apoptosis, thereby maintaining control of the immune response.
- It plays a fundamental role in numerous metabolic and biochemical reactions such as DNA synthesis and repair, protein synthesis, prostaglandin synthesis, amino acid transport and enzyme activation. Thus, every system in the body can be affected by the state of the glutathione system, especially the immune system, the nervous system, the gastrointestinal system and the lungs.[4]
Function in animals
GSH is known as a substrate in both conjugation reactions and reduction reactions, catalyzed by glutathione S-transferase enzymes in cytosol, microsomes, and mitochondria. However, it is also capable of participating in non-enzymatic conjugation with some chemicals, as in the case of N-acetyl-p-benzoquinone imine (NAPQI), the reactive cytochrome P450-reactive metabolite formed by paracetamol (or acetaminophen as it is known in the US), that becomes toxic when GSH is depleted by an overdose of acetaminophen.
Glutathione conjugates to NAPQI and helps to detoxify it, in this capacity protects cellular protein thiol groups, which would otherwise become covalently modified; when all GSH has been spent, NAPQI begins to react with the cellular proteins, killing the cells in the process. The preferred treatment for an overdose of this painkiller is the administration (usually in atomized form) of N-acetyl-L-cysteine, which is processed by cells to L-cysteine and used in the de novo synthesis of GSH.
Glutathione (GSH) participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase. It is also important as a hydrophilic molecule that is added to lipophilic toxins and waste in the liver during biotransformation before they can become part of the bile. Glutathione is also needed for the detoxification of methylglyoxal, a toxin produced as a by-product of metabolism.
This detoxification reaction is carried out by the glyoxalase system. Glyoxalase I (EC 4.4.1.5) catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-lactoyl-glutathione. Glyoxalase II (EC 3.1.2.6) catalyzes the hydrolysis of S-D-lactoyl-glutathione to glutathione and D-lactic acid.
Glutathione has recently been used as an inhibitor of melanin in the cosmetics industry. In countries like the Philippines, this product is sold as a whitening soap. Glutathione competitively inhibits melanin synthesis in the reaction of tyrosinase and L-DOPA by interrupting L-DOPA's ability to bind to tyrosinase during melanin synthesis. The inhibition of melanin synthesis was reversed by increasing the concentration of L-DOPA, but not by increasing tyrosinase. Although the synthesized melanin was aggregated within 1 h, the aggregation was inhibited by the addition of glutathione. These results indicate that glutathione inhibits the synthesis and agglutination of melanin by interrupting the function of L-DOPA. "[18]
Function in plants
In plants glutathione is crucial for biotic and abiotic stress management. It is a pivotal component of the glutathione-ascorbate cycle, a system that reduces poisonous hydrogen peroxide.[19] It is the precursor of phytochelatins, glutathione oligomeres which chelates heavy metals such as cadmium.[20] Glutathione is required for efficient defence against plant pathogens such as Pseudomonas syringae and Phytophthora brassicae.[21] APS reductase, an enzyme of the sulfur assimilation pathway uses glutathione as electron donor. Other enzymes using glutathione as substrate are glutaredoxin, these small oxidoreductases are involved in flower development, salicylic acid and plant defence signalling.[22]
Supplementation
Raising GSH levels through direct supplementation of glutathione is difficult. Research suggests that glutathione taken orally is not well absorbed across the gastrointestinal tract. In a study of acute oral administration of a very large dose (3 grams) of oral glutathione, Witschi and coworkers found that "it is not possible to increase circulating glutathione to a clinically beneficial extent by the oral administration of a single dose of 3 g of glutathione."[23][24]
However, plasma and liver GSH concentrations can be raised by administration of certain supplements that serve as GSH precursors. N-acetylcysteine, commonly referred to as NAC, is the most bioavailable precursor of glutathione.[25][26] Other supplements, including S-adenosylmethionine (SAMe)[27][28][29] and whey protein[30][31][32][33][34][35] have also been shown to increase glutathione content within the cell.
NAC is available both as a drug and as a generic supplement. Alpha lipoic acid has also been shown to restore intracellular glutathione.[36][37] Melatonin has been shown to stimulate a related enzyme, glutathione peroxidase,[38] and silymarin, an extract of the seeds of the milk thistle plant (Silybum marianum') 'has also demonstrated an ability to replenish glutathione levels.[39][40]
Low glutathione is also strongly implicated in wasting and negative nitrogen balance,[41] notably as seen in cancer, AIDS, sepsis, trauma, burns and even athletic overtraining. Glutathione supplementation can oppose this process and in AIDS, for example, result in improved survival rates.[42]
Cancer
Preliminary results indicate that glutathione changes the level of reactive oxygen species in isolated cells grown in a laboratory,[43] [44] which may reduce cancer development.[45] [46] None of these tests were performed in humans.
However, once a cancer has already developed, by conferring resistance to a number of chemotherapeutic drugs, elevated levels of glutathione in tumour cells are able to protect cancerous cells in bone marrow, breast, colon, larynx and lung cancers.[47]
Pathology
Excess glutamate at synapses, which may be released in conditions such as traumatic brain injury, can prevent the uptake of cysteine, a necessary building block of glutathione. Without the protection from oxidative injury afforded by glutathione, cells may be damaged or killed.[48]
Methods to determine glutathione
Reduced glutathione may be visualized using Ellman's reagent or bimane-derivates such as monobromobimane. The monobromobimane method is more sensitive, in this procedure cells are lysed and thiols extracted using a HCl buffer. Subsequently are the thiols reduced with dithiothreitol (DTT) and labelled by monobromobimane. Monobromobimane becomes fluorescent after binding to GSH. The thiols are then separated by HPLC and the fluorescence quantified with a fluorescence detector. Bimane may also be used to quantify glutathione in vivo. The quantification is done by confocal laser scanning microscopy after application of the dye to living cells.[49] Another approach, which allows to measure the glutathione redox potential at a high spatial and temporal resolution in living cells is based on redox imaging using the redox-sensitive green fluorescent protein (roGFP).[50]
See also
- Glutathione synthetase deficiency
- Ophthalmic acid
- roGFP, a tool to measure the cellular glutathione redox potential
- Glutathione-ascorbate cycle
- Bacterial glutathione transferase
References
- ↑ Merck Index, 11th Edition, 4369.
- ↑ Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (October 2003). "The changing faces of glutathione, a cellular protagonist". Biochem Pharmacol. 66 (8): 1499–503. doi:10.1016/S0006-2952(03)00504-5. PMID 14555227. http://linkinghub.elsevier.com/retrieve/pii/S0006295203005045.
- ↑ Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, Federici G (October 2003). "Determination of blood total, reduced, and oxidized glutathione in pediatric subjects". Clin. Chem. 47 (8): 1467–1469. PMID 11468240. http://www.clinchem.org/cgi/content/extract/47/8/1467.
- ↑ 4.0 4.1 http://www.drugs.com/pdr/immunocal-powder-sachets.html
- ↑ Dalton, TP; et al., MZ; Yang, Y; Shertzer, HG; Nebert, DW (2000). "Knockout of the Mouse Glutamate Cysteine Ligase Catalytic Subunit (Gclc) Gene: Embryonic Lethal When Homozygous, and Proposed Model for Moderate Glutathione Deficiency When Heterozygous". Biochem Biophys Res Commun. 279 (2): 324. doi:10.1006/bbrc.2000.3930. PMID 11118286.
- ↑ Yang Y; Dieter, MZ; Chen, Y; Shertzer, HG; Nebert, DW; Dalton, TP (2002). "Initial Characterization of the Glutamate-Cysteine Ligase Modifier Subunit Gclm(-/-) Knockout Mouse. NOVEL MODEL SYSTEM FOR A SEVERELY COMPROMISED OXIDATIVE STRESS RESPONSE". J Biol Chem 277 (51): 49446. doi:10.1074/jbc.M209372200. PMID 12384496.
- ↑ Giordano, G; Afsharinejad, Z; Guizzetti, M; Vitalone, A; Kavanagh, TJ; Costa, LG (2007). "Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency". Toxicol Appl Pharmacol 219 (2-3): 181. doi:10.1016/j.taap.2006.09.016. PMID 17084875.
- ↑ McConnachie LA, Mohar I, Hudson FN (October 2007). "Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice". Toxicol Sci. 99 (2): 628–36. doi:10.1093/toxsci/kfm165. PMID 17584759.
- ↑ Chen, Y; Yang, Y; Miller, ML; Shen, D; Shertzer, HG; Stringer, KF; Wang, B; Schneider, SN et al. (2007). "Hepatocyte-specificGclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure". Hepatology 45 (5): 1118. doi:10.1002/hep.21635. PMID 17464988.
- ↑ Hothorn M, Wachter A, Gromes R, Stuwe T, Rausch T, Scheffzek K (September 2006). "Structural basis for the redox control of plant glutamate cysteine ligase.". J Biol Chem 15 (37): 27557–65. doi:10.1074/jbc.M602770200. PMID 16766527.
- ↑ Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM (August 2007). "Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana.". Plant Cell 19 (8): 2653–61. doi:10.1105/tpc.107.052597. PMID 17766407.
- ↑ Wachter A, Wolf S, Steininger H, Bogs J, Rausch T. (January 2005). "Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae.". Plant J 41 (1): 15–30. doi:10.1111/j.1365-313X.2004.02269.x. PMID 15610346.
- ↑ Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer AJ (March 2008). "Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development.". Plant J 53 (6): 999–1012. doi:10.1111/j.1365-313X.2007.03389.x. PMID 18088327.
- ↑ Shelley D. Copley and Jasvinder K. Dhillon (2002). "Lateral gene transfer and parallel evolution in the history of glutathione biosynthesis genes" (free full text). Genome biology 3: research0025.1. doi:10.1186/gb-2002-3-5-research0025. http://genomebiology.com/2002/3/5/RESEARCH/0025.
- ↑ Grill D, Tausz T, De Kok LJ (2001). Significance of glutathione in plant adaptation to the environment. Springer. ISBN 1402001789. http://books.google.com/?id=aX2eJf1i67IC&pg=PA13.
- ↑ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-43W1DBV-1&_user=10&_coverDate=03%2F18%2F1996&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=1422632827&_rerunOrigin=google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5e6cae45d818742c03d3566acbe027f4
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC22706/
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18670186
- ↑ Noctor G, Foyer CH (June 1998). "ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control". Annu Rev Plant Physiol Plant Mol Biol 49: 249–279. doi:10.1146/annurev.arplant.49.1.249. PMID 15012235.
- ↑ Suk-Bong Ha, Aaron P. Smith, Ross Howden, Wendy M. Dietrich, Sarah Bugg, Matthew J. O'Connell, Peter B. Goldsbrough, and Christopher S. Cobbett (1999). "Phytochelatin Synthase Genes from Arabidopsis and the Yeast Schizosaccharomyces pombe". Plant Cell 11 (6): 1153–1164. doi:10.1105/tpc.11.6.1153. PMID 10368185. PMC 144235. http://www.plantcell.org/cgi/content/full/11/6/1153.
- ↑ Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F (January 2007). "Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis.". Plant J. 49 (1): 159–72. doi:10.1111/j.1365-313X.2006.02938.x. PMID 17144898.
- ↑ Rouhier N, Lemaire SD, Jacquot JP (2008). "The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation". Annu Rev Plant Biol 59: 143–66. doi:10.1146/annurev.arplant.59.032607.092811. PMID 18444899.
- ↑ Witschi A, Reddy S, Stofer B, Lauterburg BH (1992). "The systemic availability of oral glutathione". Eur J Clin Pharmacol. 43 (6): 667–9. doi:10.1007/BF02284971. PMID 1362956.
- ↑ AIDS Line Update
- ↑ Acetylcysteine and glutathione, PubMed
- ↑ Gross CL, Innace JK, Hovatter RC, Meier HL, Smith WJ (1993). "Biochemical manipulation of intracellular glutathione levels influences cytotoxicity to isolated human lymphocytes by sulfur mustard". Cell Biol. Toxicol. 9 (3): 259–67. doi:10.1007/BF00755604. PMID 8299004.
- ↑ Liber CS (November 2002). "S-Adenosyl-L-methionine: its role in the treatment of liver disorders". Am J Clin Nutr. 76 (5): 1183S–1187S. PMID 12418503.
- ↑ Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, Carrieri V, Albano O. (May 1989). "Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease". Scand J Gastroenterol. 24 (9): 407–15. doi:10.3109/00365528909093067. PMID 2781235.
- ↑ Loguercio C, Nardi G, Argenzio F, Aurilio C, Petrone E, Grella A, Del Vecchio Blanco C, Coltorti M. (September 1994). "Effect of S-adenosyl-L-methionine administration on red blood cell cysteine and glutathione levels in alcoholic patients with and without liver disease". Alcohol Alcohol. 29 (5): 597–604. PMID 7811344.
- ↑ Micke P, Beeh KM, Schlaak JF, Buhl R (February 2001). "Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients". Eur. J. Clin. Invest. 31 (2): 171–8. doi:10.1046/j.1365-2362.2001.00781.x. PMID 11168457.
- ↑ Moreno YF, Sgarbieri VC, da Silva MN, Toro AA, Vilela MM (February 2006). "Features of whey protein concentrate supplementation in children with rapidly progressive HIV infection". J. Trop. Pediatr. 52 (1): 34–8. doi:10.1093/tropej/fmi074. PMID 16014759.
- ↑ Grey V, Mohammed SR, Smountas AA, Bahlool R, Lands LC (December 2003). "Improved glutathione status in young adult patients with cystic fibrosis supplemented with whey protein". J. Cyst. Fibros. 2 (4): 195–8. doi:10.1016/S1569-1993(03)00097-3. PMID 15463873.
- ↑ Micke P, Beeh KM, Buhl R (February 2002). "Effects of long-term supplementation with whey proteins on plasma glutathione levels of HIV-infected patients". Eur J Nutr 41 (1): 12–8. doi:10.1007/s003940200001. PMID 11990003.
- ↑ Bounous G, Baruchel S, Falutz J, Gold P (June 1993). "Whey proteins as a food supplement in HIV-seropositive individuals". Clin Invest Med 16 (3): 204–9. PMID 8365048.
- ↑ Bounous G, Gold P (August 1991). "The biological activity of undenatured dietary whey proteins: role of glutathione". Clin Invest Med 14 (4): 296–309. PMID 1782728.
- ↑ Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009). "Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential". Biochim Biophys Acta. 1790 (Aug 4): 1149–60. doi:10.1016/j.bbagen.2009.07.026. PMID 19664690.
- ↑ Busse E, Zimmer G, Schopohl B, Kornhuber B. (1992). "Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo". Arzneimittelforschung. 42 (6): 829–31. PMID 1418040.
- ↑ Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, Poeggeler B. (1995). "Melatonin stimulates brain glutathione peroxidase activity". Neurochem Int. 26 (5): 497–502. doi:10.1016/0197-0186(94)00154-M. PMID 7492947.
- ↑ Nencini C, Giorgi G, Micheli L (2007). "Protective effect of silymarin on oxidative stress in rat brain". Phytomedicine. 14 (2-3): 129–35. doi:10.1016/j.phymed.2006.02.005. PMID 16638633.
- ↑ Valenzuela A, Aspillaga M, Vial S, Guerra R (1989). "Selectivity of silymarin on the increase of the glutathione content in different tissues of the rat". Planta Med. 55 (5): 420–2. doi:10.1055/s-2006-962056. PMID 2813578.
- ↑ Dröge W, Holm E. (1997). "Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction". FASEB J. 11 (13): 1077–89. PMID 9367343.
- ↑ Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA (1997). "Glutathione deficiency is associated with impaired survival in HIV disease". Proc Natl Acad Sci USA. 94 (5): 1967–72. doi:10.1073/pnas.94.5.1967. PMID 9050888.
- ↑ Han YH, Park WH (Aug 2009). "The effects of N-acetyl cysteine, buthionine sulfoximine, diethyldithiocarbamate or 3-amino-1,2,4-triazole on antimycin A-treated Calu-6 lung cells in relation to cell growth, reactive oxygen species and glutathione". Oncol Rep 22 (2): 385–91. PMID 19578781.
- ↑ Chow HH, Hakim IA (Aug 2007). "Modulation of human glutathione s-transferases by polyphenone intervention". Cancer Epidemiol Biomarkers Prev. 16 (8): 1662–6. doi:10.1158/1055-9965.EPI-06-0830. PMID 17684143.
- ↑ WebMD: Whey Protein May Prevent Prostate Cancer
- ↑ Glutathione Information on MedicineNet.com (a WebMD feature)
- ↑ Balendiran GK, Dabur R, Fraser D. (Nov-Dec 2004). "The role of glutathione in cancer". Cell Biochem Funct 22 (6): 343–52. doi:10.1002/cbf.1149. PMID 15386533.
- ↑ Pereira C.F, de Oliveira C.R. (Jul 2000). "Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis". Neuroscience Research 37 (3): 227–36. doi:10.1016/S0168-0102(00)00124-3. PMID 10940457.
- ↑ Meyer AJ, May MJ, Fricker M (2001). "Quantitative in vivo measurement of glutathione in Arabidopsis cells.". Plant J 27 (1): 67–78. doi:10.1046/j.1365-313x.2001.01071.x. PMID 11489184.
- ↑ Meyer, A.J., Brach, T., Marty, L., Kreye, S., Rouhier, N., Jacquot, J.P., and Hell, R. (2007). "Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer.". Plant J 52 (5): 973–86. doi:10.1111/j.1365-313X.2007.03280.x. PMID 17892447.
Related research
- Drevet JR (May 2006). "The antioxidant glutathione peroxidase family and spermatozoa: a complex story". Mol Cell Endocrinol. 250 (1-2): 70–9. doi:10.1016/j.mce.2005.12.027. PMID 16427183.
- The Role of Glutathione in Cell Defense.
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (1 March 2004). "Glutathione metabolism and its implications for health". J Nutr. 134 (3): 489–92. PMID 14988435. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=14988435.
|
|||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||