Glucagon
| edit |
| Glucagon | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1d0r. |
|||||||||||||
|
|||||||||||||
| Identifiers | |||||||||||||
| Symbols | GCG; GLP1; GLP2; GRPP | ||||||||||||
| External IDs | OMIM: 138030 MGI: 95674 HomoloGene: 1553 GeneCards: GCG Gene | ||||||||||||
|
|||||||||||||
| RNA expression pattern | |||||||||||||
 |
|||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 2641 | 14526 | |||||||||||
| Ensembl | ENSG00000115263 | ENSMUSG00000000394 | |||||||||||
| UniProt | P01275 | P55095 | |||||||||||
| RefSeq (mRNA) | NM_002054 | NM_008100 | |||||||||||
| RefSeq (protein) | NP_002045 | NP_032126 | |||||||||||
| Location (UCSC) | Chr 2: 162.71 - 162.71 Mb |
Chr 2: 62.28 - 62.28 Mb |
|||||||||||
| PubMed search | [1] | [2] | |||||||||||
Glucagon is a hormone, secreted by the pancreas, that raises blood glucose levels. Its effect is opposite that of insulin, which lowers blood glucose levels.[1] The pancreas releases glucagon when blood glucose levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream. Glucagon also stimulates the release of insulin, so that glucose can be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels at the right level.
Contents |
Function
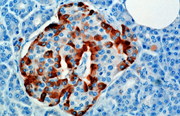
Glucagon helps maintain the level of glucose in the blood.
Glucose is stored in the liver in the form of glycogen, which is a starch-like polymer chain made up of glucose molecules. Liver cells (hepatocytes) have glucagon receptors. When glucagon binds to the glucagon receptors, the liver cells convert the glycogen polymer into individual glucose molecules, and release them into the bloodstream, in a process known as glycogenolysis. As these stores become depleted, glucagon then encourages the liver to synthesize additional glucose by gluconeogenesis.
Glucagon also regulates the rate of glucose production through lipolysis.
Glucagon production appears to be dependent on the central nervous system through pathways that are yet to be defined. It has been reported that in invertebrate animals eyestalk removal can affect glucagon production. Excising the eyestalk in young crayfish produces glucagon-induced hyperglycemia. [2]
Mechanism of action
Glucagon binds to the glucagon receptor, a G protein-coupled receptor located in the plasma membrane. The conformation change in the receptor activates G proteins, a heterotrimeric protein with α, β, and γ subunits. When the G protein interacts with the receptor, it undergoes a conformational change that results in the replacement of the GDP molecule that was bound to the α subunit with a GTP molecule. This substitution results in the releasing of the α subunit from the β and γ subunit. The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase.
Adenylate cyclase manufactures cAMP (cyclic AMP), which activates protein kinase A (cAMP-dependent protein kinase). This enzyme, in turn, activates phosphorylase kinase, which, in turn, phosphorylates glycogen phosphorylase, converting into the active form called phosphorylase A. Phosphorylase A is the enzyme responsible for the release of glucose-1-phosphate from glycogen polymers.
History
In the 1920s, Kimball and Murlin studied pancreatic extracts and found an additional substance with hyperglycemic properties. They described glucagon in 1923.[3] The amino acid sequence of glucagon was described in the late-1950s.[4] A more complete understanding of its role in physiology and disease was not established until the 1970s, when a specific radioimmunoassay was developed.
Structure
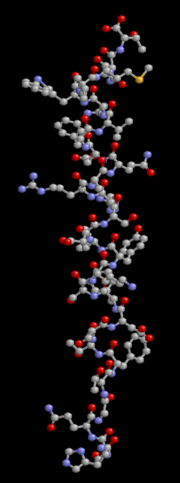
Glucagon is a 29-amino acid polypeptide. Its primary structure in humans is: NH2-His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser- Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu- Met-Asn-Thr-COOH.
The polypeptide has a molecular weight of 3485 daltons. Glucagon is a peptide (non-steroid) hormone.
Physiology
Production
The hormone is synthesized and secreted from alpha cells (α-cells) of the islets of Langerhans, which are located in the endocrine portion of the pancreas. In rodents, the alpha cells are located in the outer rim of the islet. Human islet structure is much less segregated, and alpha cells are distributed throughout the islet.
Regulatory mechanism
Increased secretion of glucagon is caused by:
- Decreased plasma glucose
- Increased catecholamines - norepinephrine and epinephrine
- Increased plasma amino acids (to protect from hypoglycemia if an all-protein meal is consumed)
- Sympathetic nervous system
- Acetylcholine
- Cholecystokinin
Decreased secretion (inhibition) of glucagon is caused by:
- Somatostatin
- Insulin
- Increased free fatty acids and ketoacids into the blood
- Increased urea production
Pathology
Abnormally-elevated levels of glucagon may be caused by pancreatic tumors such as glucagonoma, symptoms of which include necrolytic migratory erythema (NME), reduced amino acids, and hyperglycemia. It may occur alone or in the context of multiple endocrine neoplasia type 1.
Uses
An injectable form of glucagon, manufactured by Eli Lilly and Company, is vital first aid in cases of severe hypoglycemia when the victim is unconscious or for other reasons cannot take glucose orally. A competing product is sold by Novo Nordisk and is branded as the GlucaGen® HypoKit®. With both products, the dose for an adult is typically 1 milligram, and the glucagon is given by intramuscular, intravenous or subcutaneous injection, and quickly raises blood glucose levels. Glucagon can also be administered intravenously at 0.25 - 0.5 unit. To use the injectable form of glucagon, it must be reconstituted prior to use, a step that requires a sterile diluent to be injected into a vial containing powdered glucagon. The reason is because the hormone glucagon is highly unstable when dissolved in solution. When dissolved in a fluid state, glucagon can form amyloid fibrils, or tightly woven chains of proteins made up of the individual glucagon peptides, and once glucagon begins to fibrilize, it becomes useless when injected, as the glucagon cannot be absorbed and used by the body. The cumbersome reconstitution process makes using glucagon cumbersome, although there are a number of products now in development from a number of companies which aim to make the product easier to use.
Anecdotal evidence suggests a benefit of higher doses of glucagon in the treatment of overdose with beta blockers; the likely mechanism of action is the increase of cAMP in the myocardium, effectively bypassing the β-adrenergic second messenger system.[5]
Glucagon acts very quickly; common side-effects include headache and nausea.
Drug interactions: Glucagon interacts only with oral anticoagulants, increasing the tendency to bleed.
Media
See also
- Insulin
- Diabetes mellitus
- Proglucagon
- Glucagon-like peptide-1
- Glucagon-like peptide-2
- Islets of Langerhans
- Pancreas
- Cortisol
References
- ↑ Reece, Jane; Campbell, Neil (2002). Biology. San Francisco: Benjamin Cummings. ISBN 0-8053-6624-5.
- ↑ RL Leinen, AJ Giannini. Effect of eyestalk removal on glucagon induced hyperglycemia in crayfish. Society for Neuroscience Abstracts. 9:604, 1983
- ↑ Kimball C, Murlin J. Aqueous extracts of pancreas III. Some precipitation reactions of insulin. J Biol Chem 1923;58:337-348. PDF fulltext.
- ↑ Bromer W, Winn L, Behrens O. The amino acid sequence of glucagon V. Location of amide groups, acid degradation studies and summary of sequential evidence. J Am Chem Soc 1957;79:2807-2810.
- ↑ White CM. A review of potential cardiovascular uses of intravenous glucagon administration. J Clin Pharmacol 1999;39:442-7. PMID 10234590.
Further reading
- Kieffer TJ, Habener JF (2000). "The glucagon-like peptides". Endocr. Rev. 20 (6): 876–913. doi:10.1210/er.20.6.876. PMID 10605628.
- Drucker DJ (2003). "Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis". Mol. Endocrinol. 17 (2): 161–71. doi:10.1210/me.2002-0306. PMID 12554744.
- Jeppesen PB (2004). "Clinical significance of GLP-2 in short-bowel syndrome". J. Nutr. 133 (11): 3721–4. PMID 14608103.
- Brubaker PL, Anini Y (2004). "Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2". Can. J. Physiol. Pharmacol. 81 (11): 1005–12. doi:10.1139/y03-107. PMID 14719035.
- Baggio LL, Drucker DJ (2005). "Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2". Best Pract. Res. Clin. Endocrinol. Metab. 18 (4): 531–54. doi:10.1016/j.beem.2004.08.001. PMID 15533774.
- Holz GG, Chepurny OG (2006). "Diabetes outfoxed by GLP-1?". Sci. STKE 2005 (268): pe2. doi:10.1126/stke.2682005pe2. PMID 15671479.
- Dunning BE, Foley JE, Ahrén B (2006). "Alpha cell function in health and disease: influence of glucagon-like peptide-1". Diabetologia 48 (9): 1700–13. doi:10.1007/s00125-005-1878-0. PMID 16132964.
- Gautier JF, Fetita S, Sobngwi E, Salaün-Martin C (2005). "Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes". Diabetes Metab. 31 (3 Pt 1): 233–42. doi:10.1016/S1262-3636(07)70190-8. PMID 16142014.
- De León DD, Crutchlow MF, Ham JY, Stoffers DA (2006). "Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus". Int. J. Biochem. Cell Biol. 38 (5-6): 845–59. doi:10.1016/j.biocel.2005.07.011. PMID 16202636.
- Beglinger C, Degen L (2007). "Gastrointestinal satiety signals in humans--physiologic roles for GLP-1 and PYY?". Physiol. Behav. 89 (4): 460–4. doi:10.1016/j.physbeh.2006.05.048. PMID 16828127.
- Stephens JW, Bain SC (2007). "Safety and adverse effects associated with GLP-1 analogues". Expert opinion on drug safety 6 (4): 417–22. doi:10.1517/14740338.6.4.417. PMID 17688385.
- Orskov C, Bersani M, Johnsen AH, et al. (1989). "Complete sequences of glucagon-like peptide-1 from human and pig small intestine". J. Biol. Chem. 264 (22): 12826–9. PMID 2753890.
- Drucker DJ, Asa S (1988). "Glucagon gene expression in vertebrate brain". J. Biol. Chem. 263 (27): 13475–8. PMID 2901414.
- Novak U, Wilks A, Buell G, McEwen S (1987). "Identical mRNA for preproglucagon in pancreas and gut". Eur. J. Biochem. 164 (3): 553–8. doi:10.1111/j.1432-1033.1987.tb11162.x. PMID 3569278.
- White JW, Saunders GF (1986). "Structure of the human glucagon gene". Nucleic Acids Res. 14 (12): 4719–30. doi:10.1093/nar/14.12.4719. PMID 3725587.
- Schroeder WT, Lopez LC, Harper ME, Saunders GF (1984). "Localization of the human glucagon gene (GCG) to chromosome segment 2q36----37". Cytogenet. Cell Genet. 38 (1): 76–9. doi:10.1159/000132034. PMID 6546710.
- Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC (1983). "Exon duplication and divergence in the human preproglucagon gene". Nature 304 (5924): 368–71. doi:10.1038/304368a0. PMID 6877358.
- Kärgel HJ, Dettmer R, Etzold G, et al. (1982). "Action of rat liver cathepsin L on glucagon". Acta Biol. Med. Ger. 40 (9): 1139–43. PMID 7340337.
- Wayman GA, Impey S, Wu Z, et al. (1994). "Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo". J. Biol. Chem. 269 (41): 25400–5. PMID 7929237.
- Unson CG, Macdonald D, Merrifield RB (1993). "The role of histidine-1 in glucagon action". Arch. Biochem. Biophys. 300 (2): 747–50. doi:10.1006/abbi.1993.1103. PMID 8382034.
|
||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||