Melanoma
| Melanoma-Skin Cancer Disease | |
|---|---|
| Classification and external resources | |
 |
|
| ICD-10 | C43. |
| ICD-9 | 172 |
| ICD-O: | M8720/3 |
| OMIM | 155600 |
| DiseasesDB | 7947 |
| MedlinePlus | 000850 |
| eMedicine | derm/257 med/1386 ent/27 plastic/456 |
| MeSH | D008545 |
Melanoma (pronounced /ˌmɛləˈnoʊmə/ (![]() listen)) is a malignant tumor of melanocytes. Such cells are found predominantly in skin, but are also found in the bowel and the eye (see uveal melanoma). Melanoma is one of the less common types of skin cancer, but causes the majority (75%) of skin cancer related deaths.[1] Melanocytes are normally present in skin, being responsible for the production of the dark pigment melanin.[2] Despite many years of intensive laboratory and clinical research, early surgical resection of thin tumors still gives the greatest chance of cure.
listen)) is a malignant tumor of melanocytes. Such cells are found predominantly in skin, but are also found in the bowel and the eye (see uveal melanoma). Melanoma is one of the less common types of skin cancer, but causes the majority (75%) of skin cancer related deaths.[1] Melanocytes are normally present in skin, being responsible for the production of the dark pigment melanin.[2] Despite many years of intensive laboratory and clinical research, early surgical resection of thin tumors still gives the greatest chance of cure.
Around 60,000 new cases of invasive melanoma are diagnosed in the United States each year, more frequently in males and in Caucasians.[3][4] It is more common in Caucasian populations living in sunny climates than in other groups, or in those who use tanning salons.[5] According to a WHO report about 48,000 melanoma related deaths occur worldwide per year.[6]
The treatment includes surgical removal of the tumor, adjuvant treatment, chemo- and immunotherapy, or radiation therapy.
Contents |
Classification
Melanoma may be divided into the following stereotypes:[7]:694-699
-
- Lentigo maligna
- Lentigo maligna melanoma
- Superficially spreading melanoma
- Acral lentiginous melanoma
- Mucosal melanoma
- Nodular melanoma
- Polypoid melanoma
- Desmoplastic melanoma
- Amelanotic melanoma
- Soft-tissue melanoma
See also:[8]
-
- Melanoma with small nevus-like cells
- Melanoma with features of a Spitz nevus
- Uveal melanoma
Signs and symptoms
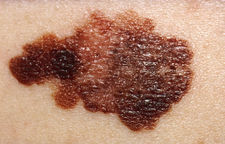
Early signs of melanoma are changes to the shape or color of existing moles or in the case of nodular melanoma the appearance of a new lump anywhere on the skin (such lesions should be referred without delay to a dermatologist). At later stages, the mole may itch, ulcerate or bleed.[9] Early signs of melanoma are summarized as " ABCDE ", where A = asymmetry, B = borders ( irregular ), C = color (variegated ), and D= diameter ( greater than 6 mm (0.24 in), about the size of a pencil erasor ).E= Evolving over time. These classifications do not however apply to the most dangerous form of melanoma nodular melanoma which has its own classifications E = elevated above the skin surface, F = firm to the touch, G = growing. Metastatic melanoma may cause non-specific paraneoplastic symptoms including loss of appetite, nausea, vomiting and fatigue. Metastasis of early melanoma is possible. However, fortunately less than a fifth of melanomas diagnosed early become metastatic.
Cause
Genetics
Familial melanoma is genetically heterogeneous,[10] and loci for familial melanoma have been identified on the chromosome arms 1p, 9p and 12q. Multiple genetic events have been related to the pathogenesis of melanoma.[11] The multiple tumor suppressor 1 (CDKN2A/MTS1) gene encodes p16INK4a - a low-molecular weight protein inhibitor of cyclin-dependent protein kinases (CDKs) - which has been localised to the p21 region of human chromosome 9.[12] Today, melanomas are diagnosed only after they become visible on the skin. In the future, however, physicians will hopefully be able detect melanomas based on a patient’s genotype, not just his or her phenotype. Recent genetic advances promise to help doctors to identify people with high-risk genotypes and to determine which of a person’s lesions have the greatest chance of becoming cancerous. A number of rare mutations, which often run in families, are known to greatly increase one’s susceptibility to melanoma. One class of mutations affects the gene CDKN2A. An alternative reading frame mutation in this gene leads to the destabilization of p53, a transcription factor involved in apoptosis and in fifty percent of human cancers. Another mutation in the same gene results in a non-functional inhibitor of CDK4, a [cyclin-dependent kinase] that promotes cell division. Mutations that cause the skin condition Xeroderma Pigmentosum (XP) also seriously predispose one to melanoma. Scattered throughout the genome, these mutations reduce a cell’s ability to repair DNA. Both CDKN2A and XP mutations are highly penetrant. Other mutations confer lower risk but are more prevalent in the population. People with mutations in the MC1R gene, for example, are two to four times more likely to develop melanoma than those with two wild-type copies of the gene. MC1R mutations are very common; in fact, all people with red hair have a mutated copy of the gene. Two-gene models of melanoma risk have already been created, and in the future, researchers hope to create genome-scale models that will allow them to predict a patient’s risk of developing melanoma based on his or her genotype. In addition to identifying high-risk patients, researchers also want to identify high-risk lesions within a given patient. Many new technologies, such as optical coherence tomography (OCT), are being developed to accomplish this. OCT allows pathologists to view 3-D reconstructions of the skin and offers more resolution than past techniques could provide. In vivo confocal microscopy and fluorescently tagged antibodies are also proving to be valuable diagnostic tools. Mutation of the MDM2 SNP309 gene is associated with increased risk of melanoma in younger women.[13]
UV radiation from tanning beds
In July 2009, the IARC released a report that categorized tanning beds as “carcinogenic to humans.” The agency, which is part of the World Health Organization (WHO), previously classified tanning beds as “probably carcinogenic.” The change comes after an analysis of more than 20 epidemiological studies indicating that people who begin using tanning devices before age 30 are 75% more likely to develop melanoma.[14]
Diagnosis

_at_thigh_Case_01.jpg)
To detect melanomas (and increase survival rates), it is recommended to learn what they look like (see "ABCD" mnemonic below), to be aware of moles and check for changes (shape, size, color, itching or bleeding) and to show any suspicious moles to a doctor with an interest and skills in skin malignancy.[15][16]
A popular method for remembering the signs and symptoms of melanoma is the mnemonic "ABCDE":
- Asymmetrical skin lesion.
- Border of the lesion is irregular.
- Color: melanomas usually have multiple colors.
- Diameter: moles greater than 6 mm are more likely to be melanomas than smaller moles.
- Enlarging: Enlarging or evolving
A weakness in this system is the D. Many melanomas present themselves as lesions smaller than 6 mm in diameter; and likely all melanomas were malignant on day 1 of growth, which is merely a dot. An astute physician will examine all abnormal moles, including ones less than 6 mm in diameter. Seborrheic keratosis may meet some or all of the ABCD criteria, and can lead to false alarms among laypeople and sometimes even physicians. An experienced doctor can generally distinguish seborrheic keratosis from melanoma upon examination, or with dermatoscopy.
Some will advocate the system "ABCDE",[17] with E for evolution. Certainly moles which change and evolve will be a concern. Alternately, some will refer to E as elevation. Elevation can help identify a melanoma, but lack of elevation does not mean that the lesion is not a melanoma. Most melanomas are detected in the very early stage, or in-situ stage, before they become elevated. By the time elevation is visible, they may have progressed to the more dangerous invasive stage.
A recent and novel method of melanoma detection is the "Ugly Duckling Sign"[18][19] It is simple, easy to teach, and highly effective in detecting melanoma. Simply, correlation of common characteristics of a person's skin lesion is made. Lesions which greatly deviate from the common characteristics are labeled as an "Ugly Duckling", and further professional exam is required. The "Little Red Riding Hood" sign[19] suggests that individuals with fair skin and light colored hair might have difficult-to-diagnose amelanotic melanomas. Extra care and caution should be rendered when examining such individuals as they might have multiple melanomas and severely dysplastic nevi. A dermatoscope must be used to detect "ugly ducklings", as many melanomas in these individuals resemble non-melanomas or are considered to be "wolves in sheep clothing".[20] These fair skinned individuals often have lightly pigmented or amelanotic melanomas which will not present easy-to-observe color changes and variation in colors. The borders of these amelanotic melanomas are often indistinct, making visual identification without a dermatoscope (dermatoscopy) very difficult.
People with a personal or family history of skin cancer or of dysplastic nevus syndrome (multiple atypical moles) should see a dermatologist at least once a year to be sure they are not developing melanoma.
Moles that are irregular in color or shape are suspicious of a malignant or a premalignant melanoma. Following a visual examination and a dermatoscopic exam,[20] used routinely by one in 4 dermatologists in the United States,[21] or an examination using other in vivo diagnostic tools, such as a confocal microscope, the doctor may biopsy the suspicious mole. If it is malignant, the mole and an area around it needs excision.
The diagnosis of melanoma requires experience, as early stages may look identical to harmless moles or not have any color at all. A skin biopsy performed under local anesthesia is often required to assist in making or confirming the diagnosis and in defining the severity of the melanoma. Amelanotic melanomas and melanomas arising in fair skinned individuals (see the "Little Red Riding Hood" sign) are very difficult to detect as they fail to show many of the characteristics in the ABCD rule, and breaks the "Ugly Duckling" sign. These melanomas are often light brown, or pink in color - and very hard to distinguish from acne scarring, insect bites, dermatofibromas, or lentigines. There is no blood test for detecting melanomas.
Surgical Treatment
Excisional skin biopsy is the management of choice. Here, the suspect lesion is totally removed with an adequate (but minimal, usually 1 or 2 mm) ellipse of surrounding skin and tissue.[22] In order not to disrupt the local lymphatic drainage, the preferred surgical margin for the initial biopsy should be narrow (1 mm). The biopsy should include the epidermal, dermal, and subcutaneous layers of the skin. This enables the histopathologist to determine the thickness of the melanoma by microscopic examination. This is described by Breslow's thickness (measured in millimeters). However, for large lesions such as suspected lentigo maligna, or for lesions in surgically difficult areas (face, toes, fingers, eyelids), a small punch biopsy in representative areas will give adequate information and will not disrupt the final staging or depth determination. In no circumstances should the initial biopsy include the final surgical margin (0.5 cm, 1.0 cm, or 2 cm), as a misdiagnosis can result in excessive scarring and morbidity from the procedure. Large initial excision will disrupt the local lymphatic drainage and can affect further lymphangiogram directed lymphnode dissection. A small punch biopsy can be utilized at any time where for logistical and personal reasons a patient refuses more invasive excisional biopsy. Small punch biopsies are minimally invasive and heal quickly, usually without noticeable scarring.
Lactate dehydrogenase (LDH) tests are often used to screen for metastases, although many patients with metastases (even end-stage) have a normal LDH; extraordinarily high LDH often indicates metastatic spread of the disease to the liver. It is common for patients diagnosed with melanoma to have chest X-rays and an LDH test, and in some cases CT, MRI, PET and/or PET/CT scans. Although controversial, sentinel lymph node biopsies and examination of the lymph nodes are also performed in patients to assess spread to the lymph nodes. A diagnosis of melanoma is supported by the presence of the S-100 protein marker.
Sometimes the skin lesion may bleed, itch, or ulcerate, although this is a very late sign. A slow-healing lesion should be watched closely, as that may be a sign of melanoma. Be aware also that in circumstances that are still poorly understood, melanomas may "regress" or spontaneously become smaller or invisible - however the malignancy is still present. Amelanotic (colorless or flesh-colored) melanomas do not have pigment and may not even be visible. Lentigo maligna, a superficial melanoma confined to the topmost layers of the skin (found primarily in older patients) is often described as a "stain" on the skin. Some patients with metastatic melanoma do not have an obvious detectable primary tumor.
Staging
Further context on cancer staging is available at TNM.
Also of importance are the "Clark level" and "Breslow's depth" which refer to the microscopic depth of tumor invasion.[23]
Melanoma stages:[24]
Stage 0: Melanoma in Situ (Clark Level I), 99.9% Survival
Stage I/II: Invasive Melanoma, 85-99% Survival
- T1a: Less than 1.00 mm primary tumor thickness, w/o Ulceration and mitosis < 1/mm2
- T1b: Less than 1.00 mm primary tumor thickness, w/Ulceration or mitoses ≥ 1/mm2
- T2a: 1.00-2.00 mm primary tumor thickness, w/o Ulceration
Stage II: High Risk Melanoma, 40-85% Survival
- T2b: 1.00-2.00 mm primary tumor thickness, w/ Ulceration
- T3a: 2.00-4.00 mm primary tumor thickness, w/o Ulceration
- T3b: 2.00-4.00 mm primary tumor thickness, w/ Ulceration
- T4a: 4.00 mm or greater primary tumor thickness w/o Ulceration
- T4b: 4.00 mm or greater primary tumor thickness w/ Ulceration
Stage III: Regional Metastasis, 25-60% Survival
- N1: Single Positive Lymph Node
- N2: 2-3 Positive Lymph Nodes OR Regional Skin/In-Transit Metastasis
- N3: 4 Positive Lymph Nodes OR Lymph Node and Regional Skin/In Transit Metastases
Stage IV: Distant Metastasis, 9-15% Survival
- M1a: Distant Skin Metastasis, Normal LDH
- M1b: Lung Metastasis, Normal LDH
- M1c: Other Distant Metastasis OR Any Distant Metastasis with Elevated LDH
Based Upon AJCC 5-Year Survival With Proper Treatment
Prevention
Minimizing exposure to sources of ultraviolet radiation (the sun and sunbeds),[25] following sun protection measures and wearing sun protective clothing (long-sleeved shirts, long trousers, and broad-brimmed hats) can offer protection. In the past it was recommended to use sunscreens with an SPF rating of 30 or higher on exposed areas as older sunscreens more effectively blocked UVA with higher SPF.[26] Currently, newer sunscreen ingredients (avobenzone, zinc, and titanium) effectively block both UVA and UVB even at lower SPFs. However, there are questions about the ability of sunscreen to prevent melanoma.[27] This controversy is well discussed in numerous review articles, and is refuted by most dermatologists.[28][29] This correlation might be due to the confounding variable that individuals who used sunscreen to prevent burn might have a higher lifetime exposure to either UVA or UVB. See Sunscreen controversy for further references and discussions. Tanning, once believed to help prevent skin cancers, actually can lead to increase incidence of melanomas.[30] Even though tanning beds emit mostly UVA, which causes tanning, it by itself might be enough to induce melanomas.
A good rule of thumb for decreasing ultraviolet light exposure is to avoid the sun between the hours of 9 a.m. and 3 p.m. or avoid the sun when your shadow is shorter than your height. These are rough rules, however, and can vary depending on locality and individual skin cancer risk.
Almost all malignant melanomas start with altering the color and appearance of normal-looking skin. This area may be a dark spot or an abnormal new mole. Other melanomas form from a mole or freckle that is already present in the skin. It can be difficult to distinguish between a malignant melanoma and a normal mole. When looking for danger signs in pigmented lesions of the skin a few simple rules are often used. The “ABCDE” list, the "ugly duckling sign", and the "red riding hood" rule are defined and discussed under the heading "Detection" earlier in this article.
“Melanoma Monday” is the kick-off of May Melanoma Month with special activities nationally and locally. Also known as National Skin Self-Examination Day. People are encouraged to examine their skin for skin cancer.[31] Since 1985, this program has helped to detect more than 188,000 suspicious lesions, including more than 21,500 suspected melanomas.
Management
Confirmation of the clinical diagnosis is either done with an excisional skin biopsy or a small full thickness sampling with a punch skin biopsy. This is usually followed with a follow up wider excision of the scar or tumor. Depending on the stage a sentinel lymph node biopsy is done as well, although controversy exists around trial evidence for this procedure. Treatment of advanced malignant melanoma is performed from a multidisciplinary approach.
Surgery
Diagnostic punch or excisional biopsies may appear to excise (and in some cases may indeed actually remove) the tumor, but further surgery is often necessary to reduce the risk of recurrence.
Complete surgical excision with adequate surgical margins and assessment for the presence of detectable metastatic disease along with short- and long-term followup is standard. Often this is done by a "wide local excision" (WLE) with 1 to 2 cm margins. Melanoma-in-situ and lentigo malignas are treated with narrower surgical margins, usually 0.2 to 0.5 cm.[32] Many surgeons consider 0.5 cm the standard of care for standard excision of melanoma-in-situ,[33] but 0.2 cm margin might be acceptable for margin controlled surgery (Mohs surgery, or the double bladed technique with margin control). The wide excision aims to reduce the rate of tumour recurrence at the site of the original lesion. This is a common pattern of treatment failure in melanoma. Considerable research has aimed to elucidate appropriate margins for excision with a general trend toward less aggressive treatment during the last decades.[34]
Mohs surgery has been reported with cure rate as low as 77%[35] and as high as 98% for melanoma-in-situ.[36] CCPDMA and the "double scalpel" peripheral margin controlled surgery is equivalent to Mohs surgery in effectiveness on this "intra-epithelial" type of melanoma.
Melanomas which spread usually do so to the lymph nodes in the region of the tumor before spreading elsewhere. Attempts to improve survival by removing lymph nodes surgically (lymphadenectomy) were associated with many complications but unfortunately no overall survival benefit. Recently the technique of sentinel lymph node biopsy has been developed to reduce the complications of lymph node surgery while allowing assessment of the involvement of nodes with tumor.[37]
Although controversial and without prolonging survival, "sentinel lymph node" biopsy is often performed, especially for T1b/T2+ tumors, mucosal tumors, ocular melanoma and tumors of the limbs. A process called lymphoscintigraphy is performed in which a radioactive tracer is injected at the tumor site in order to localize the "sentinel node(s)". Further precision is provided using a blue tracer dye and surgery is performed to biopsy the node(s). Routine H&E staining, and immunoperoxidase staining will be adequate to rule out node involvement. PCR tests on nodes, usually performed to test for entry into clinical trials, now demonstrate that many patients with a negative SLN actually had a small number of positive cells in their nodes. Alternatively, a fine-needle aspiration may be performed and is often used to test masses.
If a lymph node is positive, depending on the extent of lymph node spread, a radical lymph node dissection will often be performed. If the disease is completely resected, the patient will be considered for adjuvant therapy.
Adjuvant treatment
High risk melanomas may require adjuvant treatment. In the United States most patients in otherwise good health will begin up to a year of high-dose interferon treatment, which has severe side effects but may improve the patient's prognosis.[38] This claim is not supported by all research at this time, and in Europe interferon is usually not used outside the scope of clinical trials.[39][40]
Metastatic melanomas can be detected by X-rays, CT scans, MRIs, PET and PET/CTs, ultrasound, LDH testing and photoacoustic detection.[41]
Chemotherapy and immunotherapy
Various chemotherapy agents are used, including dacarbazine (also termed DTIC), immunotherapy (with interleukin-2 (IL-2) or interferon (IFN)) as well as local perfusion are used by different centers. They can occasionally show dramatic success, but the overall success in metastatic melanoma is quite limited.[42] IL-2 (Proleukin) is the first new therapy approved for the treatment of metastatic melanoma in 20 years. Studies have demonstrated that IL-2 offers the possibility of a complete and long-lasting remission in this disease, although only in a small percentage of patients.[43] A number of new agents and novel approaches are under evaluation and show promise.[44] Clinical trial participation should be considered the standard of care for metastatic melanoma.[45]
In 2005, a phase III clinical trial for a melanoma vaccine was halted after showing little benefit compared to placebo. One of the most promising current experimental treatment approaches, also an immunotherapy, is OncoVEX GM-CSF (BioVex Inc, Woburn, MA) which is currently in Phase 3 clinical trials following a very high level of efficacy having been observed in Phase 2.
Lentigo maligna treatment
Standard excision is still being done by most surgeons. Unfortunately, the recurrence rate is exceedingly high (up to 50%). This is due to the ill defined visible surgical margin, and the facial location of the lesions (often forcing the surgeon to use a narrow surgical margin). The narrow surgical margin used, combined with the limitation of the standard bread loafing technique of fixed tissue histology - result in a high "false negative" error rate, and frequent recurrences. Margin controlled (peripheral margins) is necessary to eliminate the false negative errors. If breadloafing is utilized, distances from sections should approach 0.1 mm to assure that the method approaches complete margin control.
Mohs surgery has been done with cure rate reported to be as low as 77%,[35] and as high as 95% by another author.[46] The "double scalpel" peripheral margin controlled excision method approximates the Mohs method in margin control, but requires a pathologist intimately familiar with the complexity of managing the vertical margin on the thin peripheral sections and staining methods.[47]
Some melanocytic nevi, and melanoma-in-situ (lentigo maligna) have resolved with an experimental treatment, imiquimod (Aldara) topical cream, an immune enhancing agent. Some dermasurgeons are combining the 2 methods: surgically excising the cancer and then treating the area with Aldara cream postoperatively for three months. Considering the very poor cure rate with standard excision, it might not be a bad idea to follow up all surgical excisions with topical imiquimod treatments.
Radiation therapy
Radiation therapy is often used after surgical resection for patients with locally or regionally advanced melanoma or for patients with unresectable distant metastases. It may reduce the rate of local recurrence but does not prolong survival.[48] Radioimmunotherapy of metastatic melanoma is currently under investigation.
Cell and Targeted Therapies
In research setting other therapies, such as adoptive cell therapy or gene therapy, may be tested.[49] Two kinds of experimental treatments developed at the National Cancer Institute (NCI), part of the National Institutes of Health in the US have been used in advanced (metastatic) melanoma with moderate success. The first treatment involves adoptive cell therapy using immune cells isolated from a patient's own melanoma tumor (TIL). These cells are grown in large numbers in a laboratory and returned to the patient after a treatment that temporarily reduces normal T cells in the patient's body. TIL Therapy following lymphodepletion can result in complete responses in highly pretreated patients[50]. The second treatment, adoptive transfer of genetically altered autologous lymphocytes, depends on delivering genes that encode so called T cell receptors (TCRs), into patient's lymphocytes. After that manipulation lymphocytes recognize and bind to certain molecules found on the surface of melanoma cells and kill them.[51] About 60% of melanomas contain a mutation in the B-Raf gene. Clinical trials with B-Raf inhibitors such as PLX4032 have resulted in clinical response rates >50%[52][53] Sutent may be effective for patients with metastatic melanoma.[54]. Many other targeted therapies are being tested in preclinical and clinical setting: for a comprehensive and publicly available collection of information on this subject, see the Targeted Therapy Database from the Melanoma Molecular Map Project [55].
Prognosis
Features that affect prognosis are tumor thickness in millimeters (Breslow's depth), depth related to skin structures (Clark level), type of melanoma, presence of ulceration, presence of lymphatic/perineural invasion, presence of tumor infiltrating lymphocytes (if present, prognosis is better), location of lesion, presence of satellite lesions, and presence of regional or distant metastasis.[56]
Certain types of melanoma have worse prognoses but this is explained by their thickness. Interestingly, less invasive melanomas even with lymph node metastases carry a better prognosis than deep melanomas without regional metastasis at time of staging. Local recurrences tend to behave similarly to a primary unless they are at the site of a wide local excision (as opposed to a staged excision or punch/shave excision) since these recurrences tend to indicate lymphatic invasion.
When melanomas have spread to the lymph nodes, one of the most important factors is the number of nodes with malignancy. Extent of malignancy within a node is also important; micrometastases in which malignancy is only microscopic have a more favorable prognosis than macrometastases. In some cases micrometastases may only be detected by special staining, and if malignancy is only detectable by a rarely employed test known as the polymerase chain reaction (PCR), the prognosis is better. Macrometastases in which malignancy is clinically apparent (in some cases cancer completely replaces a node) have a far worse prognosis, and if nodes are matted or if there is extracapsular extension, the prognosis is still worse.
When there is distant metastasis, the cancer is generally considered incurable. The five year survival rate is less than 10%.[24] The median survival is 6 to 12 months. Treatment is palliative, focusing on life-extension and quality of life. In some cases, patients may live many months or even years with metastatic melanoma (depending on the aggressiveness of the treatment). Metastases to skin and lungs have a better prognosis. Metastases to brain, bone and liver are associated with a worse prognosis.
There is not enough definitive evidence to adequately stage, and thus give a prognosis for ocular melanoma and melanoma of soft parts, or mucosal melanoma (e.g. rectal melanoma), although these tend to metastasize more easily. Even though regression may increase survival, when a melanoma has regressed, it is impossible to know its original size and thus the original tumor is often worse than a pathology report might indicate.
Epidemiology
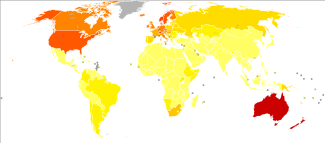
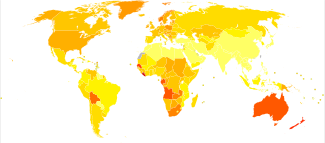
Generally, an individual's risk for developing melanoma depends on two groups of factors: intrinsic and environmental.[59] "Intrinsic" factors are generally an individual's family history and inherited genotype, while the most relevant environmental factor is sun exposure.
Epidemiologic studies suggest that exposure to ultraviolet radiation (UVA[60] and UVB) is one of the major contributors to the development of melanoma. UV radiation causes damage to the DNA of cells, typically thymine dimerization, which when unrepaired can create mutations in the cell's genes. When the cell divides, these mutations are propagated to new generations of cells. If the mutations occur in protooncogenes or tumor suppressor genes, the rate of mitosis in the mutation-bearing cells can become uncontrolled, leading to the formation of a tumor. Data from patients suggest that aberrant levels of Activating Transcription Factor in the nucleus of melanoma cells are associated with increased metastatic activity of melanoma cells;[61][62][63] studies from mice on skin cancer tend to confirm a role for Activating Transcription Factor-2 in cancer progression.[64][65] Occasional extreme sun exposure (resulting in "sunburn") is causally related to melanoma.[66] Melanoma is most common on the back in men and on legs in women (areas of intermittent sun exposure). The risk appears to be strongly influenced by socio-economic conditions rather than indoor versus outdoor occupations; it is more common in professional and administrative workers than unskilled workers.[67][68] Other factors are mutations in or total loss of tumor suppressor genes. Use of sunbeds (with deeply penetrating UVA rays) has been linked to the development of skin cancers, including melanoma.[69]
Possible significant elements in determining risk include the intensity and duration of sun exposure, the age at which sun exposure occurs, and the degree of skin pigmentation. Exposure during childhood is a more important risk factor than exposure in adulthood. This is seen in migration studies in Australia[70] where people tend to retain the risk profile of their country of birth if they migrate to Australia as an adult. Individuals with blistering or peeling sunburns (especially in the first twenty years of life) have a significantly greater risk for melanoma. This does not mean that sunburn is the cause of melanoma. Instead it is merely statistically correlated. The cause is the exaggerated UV-exposure. It has been shown that sunscreen - while preventing the sunburn - does not protect mice, injected with melanoma cells a day after UV exposure, from developing melanoma.[71]
Fair and red-headed people, persons with multiple atypical nevi or dysplastic nevi and persons born with giant congenital melanocytic nevi are at increased risk.[72]
A family history of melanoma greatly increases a person's risk because mutations in CDKN2A, CDK4 and several other genes have been found in melanoma-prone families.[73] Patients with a history of one melanoma are at increased risk of developing a second primary tumour.[74]
The incidence of melanoma has increased in the recent years, but it is not clear to what extent changes in behavior, in the environment, or in early detection are involved.[75]
To understand how sunscreen can reduce sunburn and at the same time cause melanoma it is necessary to distinguish between direct DNA damage and indirect DNA damage. Genetic analysis has shown that 92% of all melanoma are caused by the indirect DNA damage.[76] Although some people believe that dark-skinned people such as those of African descent cannot get sunburns, they are in fact susceptible, and may sunscreen accordingly, as sunscreen has been proven to protect against other cancers such as squamous cell carcinoma and basal cell carcinoma.[77]
History
Although melanoma is not a new disease, evidence for its occurrence in antiquity is rather scarce. However, one example lies in a 1960s examination of nine Peruvian Inca mummies, radiocarbon dated to be approximately 2400 years old, which showed apparent signs of melanoma: melanotic masses in the skin and diffuse metastases to the bones.[78]
John Hunter is reported to be the first to operate on metastatic melanoma in 1787. Although not knowing precisely what it was, he described it as a "cancerous fungous excrescence". The excised tumor was preserved in the Hunterian Museum of the Royal College of Surgeons of England. It was not until 1968 that microscopic examination of the specimen revealed it to be an example of metastatic melanoma.[79]
The French physician René Laennec was the first to describe melanoma as a disease entity. His report was initially presented during a lecture for the Faculté de Médecine de Paris in 1804 and then published as a bulletin in 1806.[80] The first English language report of melanoma was presented by an English general practitioner from Stourbridge, William Norris in 1820.[81] In his later work in 1857 he remarked that there is a familial predisposition for development of melanoma (Eight Cases of Melanosis with Pathological and Therapeutical Remarks on That Disease).
The first formal acknowledgment of advanced melanoma as untreatable came from Samuel Cooper in 1840. He stated that the only chance for benefit depends upon the early removal of the disease ...'[82] More than one and a half centuries later this situation remains largely unchanged.
Research
One important pathway in melanin synthesis involves the transcription factor MITF. The MITF gene is highly conserved and is found in people, mice, birds, and even fish. MITF production is regulated via a fairly straightforward pathway. UV radiation causes increased expression of transcription factor p53 in keratinocytes, and p53 causes these cells to produce melanocyte-stimulating hormone (MSH), which binds to melanocortin 1 receptors (MC1R) on melanocytes. Ligand-binding at MC1R receptors activates adenylate cyclases, which produce cAMP, which activates CREB, which promote MITF expression. The targets of MITF include p16 (a CDK inhibitor) and Bcl2, a gene essential to melanocyte survival. It is often difficult to design drugs that interfere with transcription factors, but perhaps new drugs will be discovered that can impede some reaction in the pathway upstream of MITF.
Studies of chromatin structure also promise to shed light on transcriptional regulation in melanoma cells. It has long been assumed that nucleosomes are positioned randomly on DNA, but murine studies of genes involved in melanin production now suggest that nucleosomes are stereotypically positioned on DNA. When a gene is undergoing transcription, its transcription start site is almost always nucleosome-free. When the gene is silent, however, nucleosomes often block the transcriptional start site, suggesting that nucleosome position may play a role in gene regulation. Finally, given the fact that melanin helps protect skin cells from UV-induced damage, new melanoma prevention strategies could involve attempts to induce melanin synthesis in individuals who would otherwise get sunburns. Redheads, for example, do not tan because they have MC1R mutations. In mice, it has been shown that the melanin production pathway can be rescued downstream of MC1R. Perhaps such a strategy will eventually be used to protect humans from melanoma.
See also
- List of cutaneous conditions
- Oncophage
- Seborrheic keratosis looks like melanoma but is benign
- Spitz nevus
- Dermatopathic lymphadenopathy
Notes
- ↑ "Early Detection and Treatment of Skin Cancer". American Family Physician. July 15, 2000. http://www.aafp.org/afp/20000715/357.html. Retrieved 2010-02-08.
- ↑ Cancer.gov, Cancer Stat Fact Sheets
- ↑ "What are the key statistics about melanoma?". American Cancer Society. 2010. http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_melanoma_50.asp?sitearea=. Retrieved 2010-02-08.
- ↑ Ries LAG, et al., eds. SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003: Tables XVI-1-9.
- ↑ Parkin D, Bray F, Ferlay J, Pisani P (2005). "Global cancer statistics, 2002". CA Cancer J Clin 55 (2): 74–108. doi:10.3322/canjclin.55.2.74. PMID 15761078.Full text
- ↑ Lucas, R. Global Burden of Disease of Solar Ultraviolet Radiation, Environmental Burden of Disease Series, July 25, 2006; No. 13. News release, World Health Organization
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ↑ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 1-4160-2999-0.
- ↑ MelanomaWarningSigns.com
- ↑ Greene MH. (1998). "The genetics of hereditary melanoma and nevi". Cancer 86 (11): 2464–2477. doi:10.1002/(SICI)1097-0142(19991201)86:11 (inactive 2009-04-03). PMID 10630172.
- ↑ Halachmi S, Gilchrest BA. (2001). "Update on genetic events in the pathogenesis of melanoma". Curr Opin Oncol 13 (2): 129–136. doi:10.1097/00001622-200103000-00008. PMID 11224711.
- ↑ CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) from Entrez Gene
- ↑ AACRjournals.org
- ↑ WHO International Agency for Research on Cancer Monograph Working Group (August 2009). "A Review of Human Carcinogens--Part D:Radiation". The Lancet Oncology 10 (8): 751–752. doi:10.1016/S1470-2045(09)70213-X.
- ↑ MelanomaFoundation.org
- ↑ Friedman R, Rigel D, Kopf A (1985). "Early detection of malignant melanoma: the role of physician examination and self-examination of the skin". CA Cancer J Clin 35 (3): 130–51. doi:10.3322/canjclin.35.3.130. PMID 3921200.
- ↑ AAD.org
- ↑ SkinCancer.org
- ↑ 19.0 19.1 Mascaro JM Jr, Mascaro JM. The dermatologist's position concerning nevi: a vision ranging from 'the ugly duckling' to 'little red riding hood'. Arch Dermatol 1998; 134:1484–5.
- ↑ 20.0 20.1 Dermnetnz.org
- ↑ Dermatoscopes.com
- ↑ Swanson N, Lee K, Gorman A, Lee H (2002). "Biopsy techniques. Diagnosis of melanoma". Dermatol Clin 20 (4): 677–80. doi:10.1016/S0733-8635(02)00025-6. PMID 12380054.
- ↑ Malignant melanoma: staging at Collaborative Hypertext of Radiology
- ↑ 24.0 24.1 Balch C, Buzaid A, Soong S, Atkins M, Cascinelli N, Coit D, Fleming I, Gershenwald J, Houghton A, Kirkwood J, McMasters K, Mihm M, Morton D, Reintgen D, Ross M, Sober A, Thompson J, Thompson J (2001). "Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma". J Clin Oncol 19 (16): 3635–48. PMID 11504745.Full text
- ↑ Autier P (2005). "Cutaneous malignant melanoma: facts about sunbeds and sunscreen". Expert Rev Anticancer Ther 5 (5): 821–33. doi:10.1586/14737140.5.5.821. PMID 16221052.
- ↑ Can Melanoma Be Prevented?
- ↑ Garland C, Garland F, Gorham E (1992). "Could sunscreens increase melanoma risk?". Am J Public Health 82 (4): 614–5. doi:10.2105/AJPH.82.4.614. PMID 1546792. PMC 1694089. http://www.ajph.org/cgi/reprint/82/4/614.
- ↑ HighBeam.com
- ↑ FindArticles.com
- ↑ Exposure to sunlamps, tanning beds, and melanoma risk Journal Cancer Causes and Control. Springer, Netherlands. ISSN 0957-5243 (Issue Volume 19, Number 7 / September, 2008. pp.659-69.
- ↑ [1]
- ↑ LabPath.com
- ↑ Moffitt.org
- ↑ Balch C, Urist M, Karakousis C, Smith T, Temple W, Drzewiecki K, Jewell W, Bartolucci A, Mihm M, Barnhill R (1993). "Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial". Ann Surg 218 (3): 262–7; discussion 267–9. doi:10.1097/00000658-199309000-00005. PMID 8373269.
- ↑ 35.0 35.1 Mikhail, G. Mohs Micrographic Surgery. 1991, Saunders, pp. 13–14
- ↑ Bene, NI, et al. Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases Dermatol Surg. 2008 May;34(5):660-4.Cure rate as high as 98% for small melanoma in situ, and as high as 95% noted for lentigo maligna variant of melanona in situ has been reported with Mohs surgery.
- ↑ Malignant-Melanoma.org
- ↑ Kirkwood J, Strawderman M, Ernstoff M, Smith T, Borden E, Blum R (1996). "Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684". J Clin Oncol 14 (1): 7–17. PMID 8558223.
- ↑ Kirkwood J, Ibrahim J, Sondak V, Richards J, Flaherty L, Ernstoff M, Smith T, Rao U, Steele M, Blum R (2000). "High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190". J Clin Oncol 18 (12): 2444–58. PMID 10856105.
- ↑ Kirkwood J, Ibrahim J, Sondak V, Ernstoff M, Ross M (2002). "Interferon alfa-2a for melanoma metastases". Lancet 359 (9310): 978–9. doi:10.1016/S0140-6736(02)08001-7. PMID 11918944.
- ↑ Weight RM, Viator JA, Dale PS, Caldwell CW, Lisle AE. (2006). "Photoacoustic detection of metastatic melanoma cells in the human circulatory system". Opt Lett. 31 (20): 2998–3000. doi:10.1364/OL.31.002998. PMID 17001379.
- ↑ Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O, Nova P, Aglione S, Taillibert S, Khayat D (2002). "Metastatic melanoma: chemotherapy". Semin Oncol 29 (5): 427–45. doi:10.1053/sonc.2002.35238. PMID 12407508.
- ↑ Buzaid A (2004). "Management of metastatic cutaneous melanoma". Oncology (Williston Park) 18 (11): 1443–50; discussion 1457–9. PMID 15609471.
- ↑ Danson S, Lorigan P (2005). "Improving outcomes in advanced malignant melanoma: update on systemic therapy". Drugs 65 (6): 733–43. doi:10.2165/00003495-200565060-00002. PMID 15819587.
- ↑ Bhatia S, Tykodi SS, Thompson JA (2009). "Treatment of Metastatic Melanoma: An Overview". Oncology 23 (6). http://www.cancernetwork.com/display/article/10165/1413720.
- ↑ Bene, NI, et al. Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases Dermatol Surg. 2008 May;34(5):660-4.
- ↑ Usefulness of the Staged Excision for Lentigo Maligna and Lentigo Maligna Melanoma: The 'Square' Procedure" (J Am Acad Dermatol 1997;37:758-63)
- ↑ Bastiaannet E, Beukema J, Hoekstra H (2005). "Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications". Cancer Treat Rev 31 (1): 18–26. doi:10.1016/j.ctrv.2004.09.005. PMID 15707701.
- ↑ Sotomayor M, Yu H, Antonia S, Sotomayor E, Pardoll D (2002). "Advances in gene therapy for malignant melanoma". Cancer Control 9 (1): 39–48. PMID 11907465.Full text (PDF)
- ↑ [2]
- ↑ Press release from the NIH
- ↑ Harmon, Amy (2010-02-21). "A Roller Coaster Chase for a Cure". The New York Times. http://www.nytimes.com/2010/02/22/health/research/22trial.html?. Retrieved 2010-05-12.
- ↑ K Flaherty, I Puzanov, J Sosman, Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol 2009;27:Suppl:461s-461s
- ↑ http://professional.cancerconsultants.com/oncology_melanoma_news.aspx?id=44063
- ↑ http://www.mmmp.org/MMMP/import.mmmp?page=targetedtherapydatabase.mmmp
- ↑ Homsi J, Kashani-Sabet M, Messina J, Daud A (2005). "Cutaneous melanoma: prognostic factors". Cancer Control 12 (4): 223–9. PMID 16258493.Full text (PDF)
- ↑ "CANCERMondial (GLOBOCAN)". GLOBOCAN. 2010. http://www-dep.iarc.fr/. Retrieved 12 August 2010.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html. Retrieved Nov. 11, 2009.
- ↑ Who is Most at Risk for Melanoma?
- ↑ Wang S, Setlow R, Berwick M, Polsky D, Marghoob A, Kopf A, Bart R (2001). "Ultraviolet A and melanoma: a review". J Am Acad Dermatol 44 (5): 837–46. doi:10.1067/mjd.2001.114594. PMID 11312434.
- ↑ Leslie MC, Bar-Eli M.Regulation of gene expression in melanoma: new approaches for treatment.J Cell Biochem. 2005 Jan 1;94(1):25-38.PMID: 15523674
- ↑ Bhoumik A, Singha N, O'Connell MJ, Ronai ZA.Regulation of TIP60 by ATF2 modulates ATM activation.J Biol Chem. 2008 Jun 20;283(25):17605-14.
- ↑ Bhoumik A, Jones N, Ronai Z.Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity.Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4222-7.
- ↑ Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V.The role of ATF-2 in oncogenesis.Bioessays. 2008 Apr;30(4):314-27.
- ↑ Huang Y, Minigh J, Miles S, Niles RM.Retinoic acid decreases ATF-2 phosphorylation and sensitizes melanoma cells to taxol-mediated growth inhibition.J Mol Signal. 2008 Feb 12;3:3.PMID: 18269766
- ↑ Oliveria S, Saraiya M, Geller A, Heneghan M, Jorgensen C (2006). "Sun exposure and risk of melanoma". Arch Dis Child 91 (2): 131–8. doi:10.1136/adc.2005.086918. PMID 16326797.
- ↑ Lee J, Strickland D (1980). "Malignant melanoma: social status and outdoor work". Br J Cancer 41 (5): 757–63. PMID 7426301.
- ↑ Pion IA, Rigel DS, Garfinkel L, Silverman MK, Kopf AW (January 1995). "Occupation and the risk of malignant melanoma". Cancer 75 (2 Suppl): 637–44. doi:10.1002/1097-0142(19950115)75:2 (inactive 2009-04-03). PMID 7804988.
- ↑ The World Health Organization recommends that no person under 18 should use a sunbed
- ↑ Khlat M, Vail A, Parkin M, Green A (1992). "Mortality from melanoma in migrants to Australia: variation by age at arrival and duration of stay". Am J Epidemiol 135 (10): 1103–13. PMID 1632422.
- ↑ Wolf P; Donawho C K; Kripke M L (1994). "Effect of Sunscreens on UV radiation-induced enhancements of melanoma in mice". J. Nat. Cancer. Inst. 86 (2): 99–105. doi:10.1093/jnci/86.2.99. PMID 8271307.
- ↑ Bliss J, Ford D, Swerdlow A, Armstrong B, Cristofolini M, Elwood J, Green A, Holly E, Mack T, MacKie R (1995). "Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE)". Int J Cancer 62 (4): 367–76. doi:10.1002/ijc.2910620402. PMID 7635560.
- ↑ Miller A, Mihm M (2006). "Melanoma". N Engl J Med 355 (1): 51–65. doi:10.1056/NEJMra052166. PMID 16822996.
- ↑ Rhodes A, Weinstock M, Fitzpatrick T, Mihm M, Sober A (1987). "Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals". JAMA 258 (21): 3146–54. doi:10.1001/jama.258.21.3146. PMID 3312689.
- ↑ Berwick M, Wiggins C (2006). "The current epidemiology of cutaneous malignant melanoma". Front Biosci 11: 1244–54. doi:10.2741/1877. PMID 16368510.
- ↑ Davies H.; Bignell G. R.; Cox C.; (June 2002). "Mutations of the BRAF gene in human cancer". Nature 417 (6892): 949–954. doi:10.1038/nature00766. PMID 12068308. http://www.nature.com/nature/journal/v417/n6892/full/nature00766.html.
- ↑ Ulrich, Claas; A. Degen, Manisha J. Patel and Eggert Stockfleth (2008). "Sunscreens in organ transplant patients". Nephrology Dialysis Transplantation 23 (6): 1805–1808. doi:10.1093/ndt/gfn292. PMID 18492979.
- ↑ Urteaga O, Pack G (1966). "On the antiquity of melanoma". Cancer 19 (5): 607–10. doi:10.1002/1097-0142(196605)19:5<607::AID-CNCR2820190502>3.0.CO;2-8. PMID 5326247.
- ↑ Bodenham D (1968). "A study of 650 observed malignant melanomas in the South-West region". Ann R Coll Surg Engl 43 (4): 218–39. PMID 5698493.
- ↑ Laennec RTH (1806). "Sur les melanoses". Bulletin de la Faculte de Medecine de Paris 1: 24–26.
- ↑ Norris, W. A case of fungoid disease., Edinb. Med. Surg. 1820, 16: 562-565.
- ↑ Cooper, Samuel (1840). First lines of theory and practice of surgery. London: Longman, Orme, Brown, Green and Longman.
External links
- Melanoma Molecular Map Project (MMMP) [3]
- Targeted Therapy Database (TTD) for Melanoma [4]
- American Cancer Society's Detailed Guide: Skin Cancer - Melanoma
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||