Rotavirus
| Rotavirus | |
|---|---|
 |
|
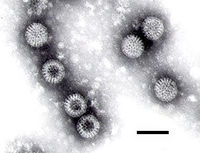
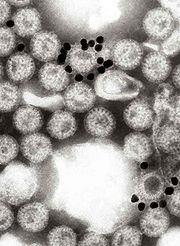
| Electron micrograph of Rotaviruses. The bar = 100 nm | |
| Virus classification | |
| Group: | Group III (dsRNA) |
| Order: | Unassigned |
| Family: | Reoviridae |
| Subfamily: | Sedoreovirinae |
| Genus: | Rotavirus |
| Type species | |
| Rotavirus A |
|
| Species | |
|
Rotavirus A |
|
| Rotaviral Gastroenteritis | |
|---|---|
| Classification and external resources | |
| ICD-10 | A08.0 |
| ICD-9 | 008.61 |
| DiseasesDB | 11667 |
| MedlinePlus | 000252 |
| eMedicine | emerg/401 |
| MeSH | D012400 |
Rotavirus is the most common cause of severe diarrhoea among infants and young children,[1] and is one of several viruses that cause infections often called stomach flu, despite having no relation to influenza. It is a genus of double-stranded RNA virus in the family Reoviridae. By the age of five, nearly every child in the world has been infected with rotavirus at least once.[2] However, with each infection, immunity develops, subsequent infections are less severe,[3] and adults are rarely affected.[4] There are five species of this virus, referred to as A, B, C, D, and E.[5] Rotavirus A, the most common, causes more than 90% of infections in humans.
The virus is transmitted by the faecal-oral route. It infects and damages the cells that line the small intestine and causes gastroenteritis. Although rotavirus was discovered in 1973[6] and accounts for up to 50% of hospitalisations for severe diarrhoea in infants and children,[7] its importance is still not widely known within the public health community, particularly in developing countries.[8] In addition to its impact on human health, rotavirus also infects animals, and is a pathogen of livestock.[9]
Rotavirus is usually an easily managed disease of childhood, but worldwide more than 500,000 children under five years of age still die from rotavirus infection each year[10] and almost two million more become severely ill.[8] In the United States, before initiation of the rotavirus vaccination programme, rotavirus caused about 2.7 million cases of severe gastroenteritis in children, almost 60,000 hospitalisations, and around 37 deaths each year.[11] Public health campaigns to combat rotavirus focus on providing oral rehydration therapy for infected children and vaccination to prevent the disease.[12]
Contents |
History
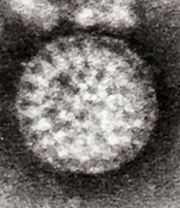
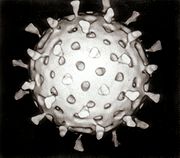
In 1943, Jacob Light and Horace Hodes proved that a filterable agent in the faeces of children with infectious diarrhoea also caused scours (livestock diarrhoea) in cattle.[13] Three decades later, preserved samples of the agent were shown to be rotavirus.[14] In the intervening years, a virus in mice[15] was shown to be related to the virus causing scours.[16] In 1973, Ruth Bishop described related viruses found in children with gastroenteritis.[6][17]
In 1974, Thomas Henry Flewett suggested the name rotavirus after observing that, when viewed through an electron microscope, a rotavirus particle looks like a wheel (rota in Latin);[18][19] the name was officially recognised by the International Committee on Taxonomy of Viruses four years later.[20] In 1976, related viruses were described in several other species of animals.[16] These viruses, all causing acute gastroenteritis, were recognised as a collective pathogen affecting humans and animals worldwide.[18] Rotavirus serotypes were first described in 1980,[21] and in the following year, rotavirus from humans was first grown in cell cultures derived from monkey kidneys, by adding trypsin (an enzyme found in the duodenum of mammals and now known to be essential for rotavirus to replicate) to the culture medium.[22] The ability to grow rotavirus in culture accelerated the pace of research, and by the mid-1980s the first candidate vaccines were being evaluated.[23]
In 1998, a rotavirus vaccine was licensed for use in the United States. Clinical trials in the United States, Finland, and Venezuela had found it to be 80 to 100% effective at preventing severe diarrhoea caused by rotavirus A, and researchers had detected no statistically significant serious adverse effects.[24][25] The manufacturer, however, withdrew it from the market in 1999, after it was discovered that the vaccine may have contributed to an increased risk for intussusception, a type of bowel obstruction, in one of every 12,000 vaccinated infants.[26] The experience provoked intense debate about the relative risks and benefits of a rotavirus vaccine.[27] In 2006, two new vaccines against rotavirus A infection were shown to be safe and effective in children,[28] and in June 2009 the World Health Organization recommended that rotavirus vaccination be included in all national immunisation programmes to provide protection against this virus.[29]
Signs and symptoms
Rotavirus gastroenteritis is a mild to severe disease characterised by vomiting, watery diarrhoea, and low-grade fever. Once a child is infected by the virus, there is an incubation period of about two days before symptoms appear.[30] Symptoms often start with vomiting followed by four to eight days of profuse diarrhoea. Dehydration is more common in rotavirus infection than in most of those caused by bacterial pathogens, and is the most common cause of death related to rotavirus infection.[31]
Rotavirus A infections can occur throughout life: the first usually produces symptoms, but subsequent infections are typically asymptomatic,[4] as the immune system provides some protection.[2] Consequently, symptomatic infection rates are highest in children under two years of age and decrease progressively towards 45 years of age.[32][33] Infection in newborn children, although common, is often associated with mild or asymptomatic disease;[34][35] the most severe symptoms tend to occur in children six months to two years of age, the elderly, and those with compromised or absent immune system functions. Due to immunity acquired in childhood, most adults are not susceptible to rotavirus; gastroenteritis in adults usually has a cause other than rotavirus, but asymptomatic infections in adults may maintain the transmission of infection in the community.[36] Symptomatic reinfections are often due to a different rotavirus A serotype.[3][37]
Transmission

Rotavirus is transmitted by the faecal-oral route, via contact with contaminated hands, surfaces and objects,[38] and possibly by the respiratory route.[1] The faeces of an infected person can contain more than 10 trillion infectious particles per gram;[4] only 10–100 of these are required to transmit infection to another person.[39]
Rotaviruses are stable in the environment and have been found in estuary samples at levels as high as 1–5 infectious particles per US gallon.[40] Sanitary measures adequate for eliminating bacteria and parasites seem to be ineffective in control of rotavirus, as the incidence of rotavirus infection in countries with high and low health standards is similar.[1]
Disease mechanisms

The diarrhoea is caused by multiple activities of the virus. Malabsorption occurs because of the destruction of gut cells called enterocytes. The toxic rotavirus protein NSP4 induces age- and calcium ion-dependent chloride secretion, disrupts SGLT1 transporter-mediated reabsorption of water, apparently reduces activity of brush-border membrane disaccharidases, and possibly activates the calcium ion-dependent secretory reflexes of the enteric nervous system.[41][42] Healthy enterocytes secrete lactase into the small intestine; milk intolerance due to lactase deficiency is a particular symptom of rotavirus infection,[43][44] which can persist for weeks.[45] A recurrence of mild diarrhoea often follows the reintroduction of milk into the child's diet, due to bacterial fermentation of the disaccharide lactose in the gut.[46]
Diagnosis and detection
Diagnosis of infection with rotavirus normally follows diagnosis of gastroenteritis as the cause of severe diarrhoea. Most children admitted to hospital with gastroenteritis are tested for rotavirus A.[47][48] Specific diagnosis of infection with rotavirus A is made by finding the virus in the child's stool by enzyme immunoassay. There are several licensed test kits on the market which are sensitive, specific and detect all serotypes of rotavirus A.[49][50] Other methods, electron microscopy and polyacrylamide gel electrophoresis, are used in research laboratories.[51] Reverse transcription-polymerase chain reaction (RT-PCR) can detect and identify all species and serotypes of human rotavirus.[52]
Treatment and prognosis
Treatment of acute rotavirus infection is nonspecific and involves management of symptoms and, most importantly, maintenance of hydration.[12] If untreated, children can die from the resulting severe dehydration.[53] Depending on the severity of diarrhoea, treatment consists of oral rehydration, during which the child is given extra water to drink that contains small amounts of salt and sugar.[54] Some infections are serious enough to warrant hospitalisation where fluids are given by intravenous drip or nasogastric tube, and the child's electrolytes and blood sugar are monitored.[47]
Rotavirus infections rarely cause other complications and for a well managed child the prognosis is excellent.[55][56] There are rare reports of complications involving the central nervous system (CNS) where rotavirus was detected in the fluid of the CNS in cases of encephalitis and meningitis,[57][58][59] and recent studies have confirmed that rotavirus infection is not always confined to the gut, but can cause viremia.[60]
Epidemiology
Rotavirus A, which accounts for more than 90% of rotavirus gastroenteritis in humans,[61] is endemic worldwide. Each year rotavirus causes millions of cases of diarrhoea in developing countries, almost 2 million resulting in hospitalisation[8] and an estimated 611,000 resulting in death.[62] In the United States alone—before initiation of the rotavirus vaccination programme[63]—over 2.7 million cases of rotavirus gastroenteritis occurred annually, 60,000 children were hospitalised and around 37 died from the results of the infection.[11] The major role of rotavirus in causing diarrhoea is not widely recognised within the public health community,[64] particularly in developing countries.[8] Almost every child has been infected with rotavirus by age five.[62] It is the leading single cause of severe diarrhoea among infants and children, being responsible for about 20% of cases, and accounts for 50% of the cases requiring hospitalisation.[8] Boys are twice as likely to be admitted to hospital as girls.[7][65] In temperate areas, rotavirus infections occur primarily in the winter, but in the tropics they occur throughout the year;[66] the difference is partly explained by seasonal changes in temperature and humidity.[67][68] The number attributable to food contamination is unknown.[69]
"Rotavirus is estimated to cause about 40 per cent of all hospital admissions due to diarrhoea among children under five years of age worldwide—leading to some 100 million episodes of acute diarrhoea each year that result in 350,000 to 600,000 child deaths."
Outbreaks of rotavirus A diarrhoea are common among hospitalised infants, young children attending day care centres, and elderly people in nursing homes. An outbreak caused by contaminated municipal water occurred in Colorado in 1981.[71] During 2005, the largest recorded epidemic of diarrhoea occurred in Nicaragua. This unusually large and severe outbreak was associated with mutations in the rotavirus A genome, possibly helping the virus escape the prevalent immunity in the population.[72] A similar large outbreak occurred in Brazil in 1977.[73]
Rotavirus B, also called adult diarrhoea rotavirus or ADRV, has caused major epidemics of severe diarrhoea affecting thousands of people of all ages in China. These epidemics occurred as a result of sewage contamination of drinking water.[74][75] Rotavirus B infections also occurred in India in 1998; the causative strain was named CAL. Unlike ADRV, the CAL strain is endemic.[76][77] To date, epidemics caused by rotavirus B have been confined to mainland China, and surveys indicate a lack of immunity to this species in the United States.[78]
Rotavirus C has been associated with rare and sporadic cases of diarrhoea in children in many countries, and outbreaks have occurred in Japan and England.[79][80]
Prevention
Because improved sanitation does not decrease the prevalence of rotaviral disease, and the rate of hospitalisations remains high, despite the use of oral rehydrating medicines, the primary public health intervention is vaccination.[81] In 2006, two vaccines against Rotavirus A infection were shown to be safe and effective in children: Rotarix by GlaxoSmithKline[82] and RotaTeq by Merck.[83] Both are taken orally and contain attenuated live virus. Rotavirus vaccines are licensed in more than 100 countries, but only 17 countries have introduced routine rotavirus vaccination.[84]
The Rotavirus Vaccine Program is a collaboration between PATH, the World Health Organization, and the U.S. Centers for Disease Control and Prevention, and is funded by the GAVI Alliance. The Program aims to reduce child morbidity and mortality from diarrhoeal disease by making a vaccine against rotavirus available for use in developing countries.[85]
On June 5, 2009, WHO announced that clinical trials of Rotarix vaccine “in high-mortality, low-socioeconomic settings of South Africa and Malawi, found that the vaccine significantly reduced severe diarrhoea episodes due to rotavirus.” WHO now recommends that rotavirus vaccine be included in all national immunization programs[86] and the incidence of rotavirus disease in the United States has declined since introduction of rotavirus vaccination for children .[63] Additional rotavirus vaccines are under development.[87]
Infections of animals
Rotaviruses infect and cause diarrhoea in young animals. They have been shown to infect mammals (for example, apes,[88] cattle,[89] pigs,[90] sheep,[9] rats,[91] cats and dogs,[92] mice,[93] horses,[94] rabbits)[95] and birds (chickens and turkeys).[96] These rotaviruses are a potential reservoir for genetic exchange with human rotaviruses. There is evidence that animal rotaviruses can infect humans, either by direct transmission of the virus or by contributing one or several RNA segments to reassortants with human strains.[97][98] Rotavirus are a pathogen of livestock and cause economic loss to farmers because of costs of treatment associated with high morbidity and mortality rates.[9]
Virology
Types of rotavirus
There are five species of rotavirus, referred to as A, B, C, D and E. Humans are primarily infected by species A, B and C, most commonly by species A. All five species cause disease in other animals.[99]
Within rotavirus A there are different strains, called serotypes.[100] As with influenza virus, a dual classification system is used, which is based on two structural proteins on the surface of the virion. The glycoprotein VP7 defines G-types and the protease-sensitive protein VP4 defines P-types. Strains are generally designated by their G serotype specificities (e.g., serotypes G1 to G4 and G9), and the P-type is indicated by a number and a letter for the P-serotype and by a number in square brackets for the corresponding P-genotype. (P-serotypes are difficult to characterize; therefore, molecular methods based on sequence analysis are often used to define the corresponding P-genotype instead. These genotypes correlate well with known P-serotypes).[28] Because the two genes that determine G-types and P-types can be passed on separately to offspring, various combinations occur in any one strain. The Wa strain is classified in full as G1P1A[8].[101]
Structure
The genome of rotavirus consists of 11 unique double helix molecules of RNA which are 18,555 nucleotides in total. Each helix, or segment, is a gene, numbered 1 to 11 by decreasing size. Each gene codes for one protein, except genes 9 and 11, which each code for two.[102] The RNA is surrounded by a three-layered icosahedral protein capsid. Viral particles are up to 76.5 nm in diameter[103][104] and are not enveloped.
Proteins
There are six viral proteins (VPs) that form the virus particle (virion). These structural proteins are called VP1, VP2, VP3, VP4, VP6 and VP7. In addition to the VPs, there are six nonstructural proteins (NSPs), that are only produced in cells infected by rotavirus. These are called NSP1, NSP2, NSP3, NSP4, NSP5 and NSP6.
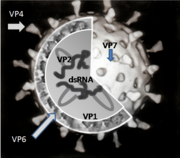
At least six of the twelve proteins encoded by the rotavirus genome bind RNA.[105] The role of these proteins play in rotavirus replication is not entirely understood; their functions are thought to be related to RNA synthesis and packaging in the virion, mRNA transport to the site of genome replication, and mRNA translation and regulation of gene expression.[106]
Structural proteins
VP1 is located in the core of the virus particle and is an RNA polymerase enzyme.[107] In an infected cell this enzyme produces mRNA transcripts for the synthesis of viral proteins and produces copies of the rotavirus genome RNA segments for newly produced virus particles.
VP2 forms the core layer of the virion and binds the RNA genome.[108]
VP3 is part of the inner core of the virion and is an enzyme called guanylyl transferase. This is a capping enzyme that catalyses the formation of the 5' cap in the post-transcriptional modification of mRNA.[109] The cap stabilises viral mRNA by protecting it from nucleic acid degrading enzymes called nucleases.
VP4 is on the surface of the virion that protrudes as a spike.[110] It binds to molecules on the surface of cells called receptors and drives the entry of the virus into the cell.[111] VP4 has to be modified by a protease enzyme (found in the gut) into VP5* and VP8* before the virus is infectious.[112] It determines how virulent the virus is and it determines the P-type of the virus.[113]
VP6 forms the bulk of the capsid. It is highly antigenic and can be used to identify rotavirus species.[4] This protein is used in laboratory tests for rotavirus A infections.[50]
VP7 is a glycoprotein that forms the outer surface of the virion. Apart from its structural functions, it determines the G-type of the strain and, along with VP4, is involved in immunity to infection.[103]
Nonstructural viral proteins
NSP1, the product of gene 5, is a nonstructural RNA-binding protein.[114]
NSP2 is an RNA-binding protein that accumulates in cytoplasmic inclusions (viroplasms) and is required for genome replication.[115][116]
NSP3 is bound to viral mRNAs in infected cells and it is responsible for the shutdown of cellular protein synthesis.[117]
NSP4 is a viral enterotoxin to induce diarrhoea and was the first viral enterotoxin discovered.[118]
NSP5 is encoded by genome segment 11 of rotavirus A and in virus-infected cells NSP5 accumulates in the viroplasm.[119]
NSP6 is a nucleic acid binding protein,[120] and is encoded by gene 11 from an out of phase open reading frame.[121]
| RNA Segment (Gene) | Size (base pairs) | Protein | Molecular weight kDa | Location | Copies per particle | Function |
|---|---|---|---|---|---|---|
| 1 | 3302 | VP1 | 125 | At the vertices of the core | <25 | RNA-dependent RNA polymerase |
| 2 | 2690 | VP2 | 102 | Forms inner shell of the core | 120 | Stimulates viral RNA replicase |
| 3 | 2591 | VP3 | 88 | At the vertices of the core | <25 | Guanylyl transferase mRNA capping enzyme |
| 4 | 2362 | VP4 | 87 | Surface spike | 120 | Cell attachment, virulence |
| 5 | 1611 | NSP1 | 59 | Nonstructural | 0 | 5'RNA binding |
| 6 | 1356 | VP6 | 45 | Inner Capsid | 780 | Structural and species-specific antigen |
| 7 | 1104 | NSP3 | 37 | Nonstructural | 0 | Enhances viral mRNA activity and shut-offs cellular protein synthesis |
| 8 | 1059 | NSP2 | 35 | Nonstructural | 0 | NTPase involved in RNA packaging |
| 9 | 1062 | VP71 VP72 | 38 and 34 | Surface | 780 | Structural and neutralisation antigen |
| 10 | 751 | NSP4 | 20 | Nonstructural | 0 | Enterotoxin |
| 11 | 667 | NSP5 NSP6 | 22 | Nonstructural | 0 | ssRNA and dsRNA binding modulator of NSP2 |
This table is based on the simian rotavirus strain SA11.[122][123][124] RNA-protein coding assignments differ in some strains.
Replication
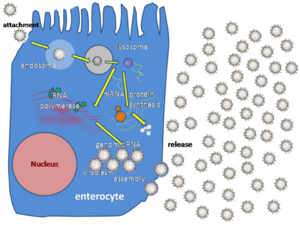
Rotaviruses replicate mainly in the gut,[125] and infect enterocytes of the villi of the small intestine, leading to structural and functional changes of the epithelium.[126] The triple protein coats make them resistant to the acidic pH of the stomach and the digestive enzymes in the gut.
The virus enter cells by receptor mediated endocytosis and form a vesicle known as an endosome. Proteins in the third layer (VP7 and the VP4 spike) disrupt the membrane of the endosome, creating a difference in the calcium concentration. This causes the breakdown of VP7 trimers into single protein subunits, leaving the VP2 and VP6 protein coats around the viral dsRNA, forming a double-layered particle (DLP).
The eleven dsRNA strands remain within the protection of the two protein shells and the viral RNA-dependent RNA polymerase creates mRNA transcripts of the double-stranded viral genome. By remaining in the core, the viral RNA evades innate host immune responses called RNA interference that are triggered by the presence of double-stranded RNA.
During the infection, rotavirus produces mRNA for both protein biosynthesis and gene replication. Most of the rotavirus proteins accumulate in viroplasm, where the RNA is replicated and the DLPs are assembled. Viroplasm is formed around the cell nucleus as early as two hours after virus infection, and consists of viral factories thought to be made by two viral nonstructural proteins: NSP5 and NSP2. Inhibition of NSP5 by RNA interference results in a sharp decrease in rotavirus replication. The DLPs migrate to the endoplasmic reticulum where they obtain their third, outer layer (formed by VP7 and VP4). The progeny viruses are released from the cell by lysis.[127][128]
See also
- Bacterial gastroenteritis
- Infant mortality
References
- ↑ 1.0 1.1 1.2 Dennehy PH (2000). "Transmission of rotavirus and other enteric pathogens in the home". Pediatr. Infect. Dis. J. 19 (10 Suppl): S103–5. doi:10.1097/00006454-200010001-00003. PMID 11052397.
- ↑ 2.0 2.1 Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM (1996). "Rotavirus infections in infants as protection against subsequent infections". N. Engl. J. Med. 335 (14): 1022–8. doi:10.1056/NEJM199610033351404. PMID 8793926. http://content.nejm.org/cgi/content/full/335/14/1022.
- ↑ 3.0 3.1 Linhares AC, Gabbay YB, Mascarenhas JD, Freitas RB, Flewett TH, Beards GM (1988). "Epidemiology of rotavirus subgroups and serotypes in Belem, Brazil: a three-year study". Ann. Inst. Pasteur Virol. 139 (1): 89–99. doi:10.1016/S0769-2617(88)80009-1. PMID 2849961.
- ↑ 4.0 4.1 4.2 4.3 Bishop RF (1996). "Natural history of human rotavirus infection". Arch. Virol. Suppl. 12: 119–28. PMID 9015109.
- ↑ ICTV Virus Taxonomy: 2009 Release
- ↑ 6.0 6.1 Bishop RF, Davidson GP, Holmes IH, Ruck BJ (1973). "Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis". Lancet 2 (7841): 1281–3. doi:10.1016/S0140-6736(73)92867-5. PMID 4127639.
- ↑ 7.0 7.1 Rheingans RD, Heylen J, Giaquinto C (2006). "Economics of rotavirus gastroenteritis and vaccination in Europe: what makes sense?". Pediatr. Infect. Dis. J. 25 (1 Suppl): S48–55. doi:10.1097/01.inf.0000197566.47750.3d. PMID 16397429.
- ↑ 8.0 8.1 8.2 8.3 8.4 Simpson E, Wittet S, Bonilla J, Gamazina K, Cooley L, Winkler JL (2007). "Use of formative research in developing a knowledge translation approach to rotavirus vaccine introduction in developing countries". BMC Public Health 7: 281. doi:10.1186/1471-2458-7-281. PMID 17919334. PMC 2173895. http://www.biomedcentral.com/1471-2458/7/281.
- ↑ 9.0 9.1 9.2 Holland RE (1 October 1990). "Some infectious causes of diarrhea in young farm animals" (PDF). Clin. Microbiol. Rev. 3 (4): 345–75. PMID 2224836. PMC 358168. http://cmr.asm.org/cgi/reprint/3/4/345.
- ↑ World Health Organization. Rotavirus vaccines position paper.
- ↑ 11.0 11.1 Fischer TK, Viboud C, Parashar U, et al. (2007). "Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003". J. Infect. Dis. 195 (8): 1117–25. doi:10.1086/512863. PMID 17357047.
- ↑ 12.0 12.1 Diggle L (2007). "Rotavirus diarrhoea and future prospects for prevention". Br. J. Nurs. 16 (16): 970–4. PMID 18026034.
- ↑ Light JS, Hodes HL (1943). "Studies on epidemic diarrhea of the new-born: Isolation of a Filtrable Agent Causing Diarrhea in Calves". Am. J. Public Health Nations Health 33 (12): 1451–4. PMID 18015921.
- ↑ Mebus CA, Wyatt RG, Sharpee RL, et al. (1 August 1976). "Diarrhea in gnotobiotic calves caused by the reovirus-like agent of human infantile gastroenteritis" (PDF). Infect. Immun. 14 (2): 471–4. PMID 184047. PMC 420908. http://iai.asm.org/cgi/reprint/14/2/471.
- ↑ Rubenstein D, Milne RG, Buckland R, Tyrrell DA (1971). "The growth of the virus of epidemic diarrhoea of infant mice (EDIM) in organ cultures of intestinal epithelium". British journal of experimental pathology 52 (4): 442–45. PMID 4998842.
- ↑ 16.0 16.1 Woode GN, Bridger JC, Jones JM, Flewett TH, Davies HA, Davis HA, White GB (1 September 1976). "Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis in children, calves, piglets, mice, and foals" (PDF). Infect. Immun. 14 (3): 804–10. PMID 965097. PMC 420956. http://iai.asm.org/cgi/reprint/14/3/804.
- ↑ Bishop RF, Davidson GP, Holmes IH, Ruck BJ (1973). "Letter: Evidence for viral gastroenteritis". N. Engl. J. Med. 289 (20): 1096–7. PMID 4742237.
- ↑ 18.0 18.1 Flewett TH, Woode GN (1978). "The rotaviruses". Arch. Virol. 57 (1): 1–23. doi:10.1007/BF01315633. PMID 77663.
- ↑ Flewett TH, Bryden AS, Davies H, Woode GN, Bridger JC, Derrick JM (1974). "Relation between viruses from acute gastroenteritis of children and newborn calves". Lancet 2 (7872): 61–3. doi:10.1016/S0140-6736(74)91631-6. PMID 4137164.
- ↑ Matthews RE (1979). "Third report of the International Committee on Taxonomy of Viruses. Classification and nomenclature of viruses". Intervirology 12 (3–5): 129–296. doi:10.1159/000149081. PMID 43850.
- ↑ Beards GM, Pilfold JN, Thouless ME, Flewett TH (1980). "Rotavirus serotypes by serum neutralisation". J. Med. Virol. 5 (3): 231–7. doi:10.1002/jmv.1890050307. PMID 6262451.
- ↑ Urasawa T, Urasawa S, Taniguchi K (1981). "Sequential passages of human rotavirus in MA-104 cells". Microbiol. Immunol. 25 (10): 1025–35. PMID 6273696.
- ↑ Vesikari T, Isolauri E, Delem A, et al. (1985). "Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic". J. Pediatr. 107 (2): 189–94. doi:10.1016/S0022-3476(85)80123-2. PMID 3894608.
- ↑ "Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm Rep 48 (RR-2): 1–20. 1999. PMID 10219046.
- ↑ Kapikian AZ (2001). "A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal". Novartis Found. Symp. 238: 153–71; discussion 171–9. doi:10.1002/0470846534.ch10. PMID 11444025.
- ↑ Bines JE (2005). "Rotavirus vaccines and intussusception risk". Curr. Opin. Gastroenterol. 21 (1): 20–5. PMID 15687880. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0267-1379&volume=21&issue=1&spage=20.
- ↑ Bines J (2006). "Intussusception and rotavirus vaccines". Vaccine 24 (18): 3772–6. doi:10.1016/j.vaccine.2005.07.031. PMID 16099078.
- ↑ 28.0 28.1 Dennehy PH (2008). "Rotavirus vaccines: an overview". Clin. Microbiol. Rev. 21 (1): 198–208. doi:10.1128/CMR.00029-07. PMID 18202442. PMC 2223838. http://cmr.asm.org/cgi/content/full/21/1/198?view=long&pmid=18202442.
- ↑ "Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations". Relevé Épidémiologique Hebdomadaire / Section D'hygiène Du Secrétariat De La Société Des Nations = Weekly Epidemiological Record / Health Section of the Secretariat of the League of Nations 84 (23): 220–36. June 2009. PMID 19499606.[1]
- ↑ Hochwald C, Kivela L (1999). "Rotavirus vaccine, live, oral, tetravalent (RotaShield)". Pediatr. Nurs. 25 (2): 203–4, 207. PMID 10532018.
- ↑ Maldonado YA, Yolken RH (1990). "Rotavirus". Baillieres Clin. Gastroenterol. 4 (3): 609–25. doi:10.1016/0950-3528(90)90052-I. PMID 1962726.
- ↑ Bernstein DI, Sander DS, Smith VE, Schiff GM, Ward RL (1991). "Protection from rotavirus reinfection: 2-year prospective study". J. Infect. Dis. 164 (2): 277–83. PMID 1649875.
- ↑ Koopman JS, Monto AS (1989). "The Tecumseh Study. XV: Rotavirus infection and pathogenicity". Am. J. Epidemiol. 130 (4): 750–9. PMID 2549788.
- ↑ Cameron DJ, Bishop RF, Veenstra AA, Barnes GL (1 July 1978). "Noncultivable viruses and neonatal diarrhea: Fifteen-month survey in a newborn special care nursery" (PDF). J. Clin. Microbiol. 8 (1): 93–8. PMID 209058. PMC 275123. http://jcm.asm.org/cgi/reprint/8/1/93.
- ↑ Grillner L, Broberger U, Chrystie I, Ransjö U (1985). "Rotavirus infections in newborns: an epidemiological and clinical study". Scand. J. Infect. Dis. 17 (4): 349–55. PMID 3003889.
- ↑ Hrdy DB (1987). "Epidemiology of rotaviral infection in adults". Rev. Infect. Dis. 9 (3): 461–9. PMID 3037675.
- ↑ De Champs C, Laveran H, Peigue-Lafeuille H, et al. (1991). "Sequential rotavirus infections: characterization of serotypes and electrophoretypes". Res. Virol. 142 (1): 39–45. doi:10.1016/0923-2516(91)90026-Y. PMID 1647052.
- ↑ Butz AM, Fosarelli P, Dick J, Cusack T, Yolken R (1993). "Prevalence of rotavirus on high-risk fomites in day-care facilities". Pediatrics 92 (2): 202–5. PMID 8393172.
- ↑ Graham DY, Dufour GR, Estes MK (1987). "Minimal infective dose of rotavirus". Arch. Virol. 92 (3–4): 261–71. doi:10.1007/BF01317483. PMID 3028333.
- ↑ Rao VC, Seidel KM, Goyal SM, Metcalf TG, Melnick JL (1 August 1984). "Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments" (PDF). Appl. Environ. Microbiol. 48 (2): 404–9. PMID 6091548. PMC 241526. http://aem.asm.org/cgi/reprint/48/2/404.
- ↑ Ball JM, Mitchell DM, Gibbons TF, Parr RD (2005). "Rotavirus NSP4: a multifunctional viral enterotoxin". Viral Immunol. 18 (1): 27–40. doi:10.1089/vim.2005.18.27. PMID 15802952.
- ↑ Lorrot M, Vasseur M (2007). "How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea?". Virol. J. 4: 31. doi:10.1186/1743-422X-4-31. PMID 17376232. PMC 1839081. http://www.virologyj.com/content/4/1/31.
- ↑ Jourdan N, Brunet JP, Sapin C, et al. (1 September 1998). "Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton". J. Virol. 72 (9): 7228–36. PMID 9696817. PMC 109945. http://jvi.asm.org/cgi/content/full/72/9/7228.
- ↑ Davidson GP, Barnes GL (1979). "Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis". Acta. Paediatr. Scand. 68 (2): 181–6. doi:10.1111/j.1651-2227.1979.tb04986.x. PMID 217231.
- ↑ Ouwehand A, Vesterlund S (2003). "Health aspects of probiotics". IDrugs 6 (6): 573–80. PMID 12811680.
- ↑ Arya SC (1984). "Rotaviral infection and intestinal lactase level". J. Infect. Dis. 150 (5): 791. PMID 6436397.
- ↑ 47.0 47.1 Patel MM, Tate JE, Selvarangan R, et al. (2007). "Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination". Pediatr. Infect. Dis. J. 26 (10): 914–9. doi:10.1097/INF.0b013e31812e52fd. PMID 17901797.
- ↑ The Pediatric ROTavirus European CommitTee (PROTECT) (2006). "The paediatric burden of rotavirus disease in Europe". Epidemiol. Infect. 134 (5): 908–16. doi:10.1017/S0950268806006091. PMID 16650331.
- ↑ Smith TF, Wold AD, Espy MJ, Marshall WF (1993). "New developments in the diagnosis of viral diseases". Infect. Dis. Clin. North Am. 7 (2): 183–201. PMID 8345165.
- ↑ 50.0 50.1 Beards GM, Campbell AD, Cottrell NR, Peiris JS, Rees N, Sanders RC, Shirley JA, Wood HC, Flewett TH (1 February 1984). "Enzyme-linked immunosorbent assays based on polyclonal and monoclonal antibodies for rotavirus detection" (PDF). J. Clin. Microbiol. 19 (2): 248–54. PMID 6321549. PMC 271031. http://jcm.asm.org/cgi/reprint/19/2/248.
- ↑ Beards GM (1988). "Laboratory diagnosis of viral gastroenteritis". Eur. J. Clin. Microbiol. Infect. Dis. 7 (1): 11–3. doi:10.1007/BF01962164. PMID 3132369.
- ↑ Fischer TK, Gentsch JR (2004). "Rotavirus typing methods and algorithms". Rev. Med. Virol. 14 (2): 71–82. doi:10.1002/rmv.411. PMID 15027000.
- ↑ Alam NH, Ashraf H (2003). "Treatment of infectious diarrhea in children". Paediatr. Drugs 5 (3): 151–65. PMID 12608880.
- ↑ Sachdev HP (1996). "Oral rehydration therapy". Journal of the Indian Medical Association 94 (8): 298–305. PMID 8855579.
- ↑ Haffejee IE (1991). "The pathophysiology, clinical features and management of rotavirus diarrhoea". Q. J. Med. 79 (288): 289–99. PMID 1649479. http://qjmed.oxfordjournals.org/cgi/reprint/79/1/289.
- ↑ Ramig RF (2007). "Systemic rotavirus infection". Expert review of anti-infective therapy 5 (4): 591–612. doi:10.1586/14787210.5.4.591. PMID 17678424.
- ↑ Goto T, Kimura H, Numazaki K, et al. (2007). "A case of meningoencephalitis associated with G1P[8] rotavirus infection in a Japanese child". Scand. J. Infect. Dis. 39 (11): 1067–70. doi:10.1080/00365540701466249. PMID 17852929.
- ↑ Kehle J, Metzger-Boddien C, Tewald F, Wald M, Schüürmann J, Enders G (2003). "First case of confirmed rotavirus meningoencephalitis in Germany". Pediatr. Infect. Dis. J. 22 (5): 468–70. doi:10.1097/00006454-200305000-00020. PMID 12797316.
- ↑ Pager C, Steele D, Gwamanda P, Driessen M (2000). "A neonatal death associated with rotavirus infection—detection of rotavirus dsRNA in the cerebrospinal fluid". S. Afr. Med. J. 90 (4): 364–5. PMID 10957919.
- ↑ Widdowson MA, Bresee JS, Gentsch JR, Glass RI (2005). "Rotavirus disease and its prevention". Curr. Opin. Gastroenterol. 21 (1): 26–31. PMID 15687881. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0267-1379&volume=21&issue=1&spage=26.
- ↑ Leung AK, Kellner JD, Davies HD (2005). "Rotavirus gastroenteritis". Adv. Ther. 22 (5): 476–87. doi:10.1007/BF02849868. PMID 16418157.
- ↑ 62.0 62.1 Parashar UD, Gibson CJ, Bresse JS, Glass RI (2006). "Rotavirus and severe childhood diarrhea". Emerging Infect. Dis. 12 (2): 304–6. PMID 16494759.
- ↑ 63.0 63.1 Centers for Disease Control and Prevention (CDC) (October 2009). "Reduction in rotavirus after vaccine introduction—United States, 2000–2009". MMWR. Morbidity and Mortality Weekly Report 58 (41): 1146–9. PMID 19847149. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5841a2.htm. Retrieved 2009-12-20.
- ↑ Rodrigo C, Salman N, Tatochenko V, Mészner Z, Giaquinto C (May 2010). "Recommendations for rotavirus vaccination: A worldwide perspective". Vaccine 28 (31): 5100–8. doi:10.1016/j.vaccine.2010.04.108. PMID 20472032.
- ↑ Ryan MJ, Ramsay M, Brown D, Gay NJ, Farrington CP, Wall PG (1996). "Hospital admissions attributable to rotavirus infection in England and Wales". J. Infect. Dis. 174 Suppl 1: S12–8. PMID 8752285.
- ↑ Cook SM, Glass RI, LeBaron CW, Ho MS (1990). "Global seasonality of rotavirus infections". Bull. World Health Organ. 68 (2): 171–7. PMID 1694734.
- ↑ Moe K, Harper GJ (1983). "The effect of relative humidity and temperature on the survival of bovine rotavirus in aerosol". Arch. Virol. 76 (3): 211–6. doi:10.1007/BF01311105. PMID 6307226.
- ↑ Moe K, Shirley JA (1982). "The effects of relative humidity and temperature on the survival of human rotavirus in faeces". Arch. Virol. 72 (3): 179–86. doi:10.1007/BF01348963. PMID 6287970.
- ↑ Koopmans M, Brown D (1999). "Seasonality and diversity of Group A rotaviruses in Europe". Acta paediatrica (Oslo, Norway : 1992). Supplement 88 (426): 14–9. doi:10.1111/j.1651-2227.1999.tb14320.x. PMID 10088906.
- ↑ UNICEF/WHO (2009) "Diarrhoea: Why children are still dying and what can be done." Retrieved 23 May 2010
- ↑ Hopkins RS, Gaspard GB, Williams FP, Karlin RJ, Cukor G, Blacklow NR (1984). "A community waterborne gastroenteritis outbreak: evidence for rotavirus as the agent". American Journal of Public Health 74 (3): 263–5. doi:10.2105/AJPH.74.3.263. PMID 6320684.
- ↑ Bucardo F, Karlsson B, Nordgren J, et al. (2007). "Mutated G4P[8] rotavirus associated with a nationwide outbreak of gastroenteritis in Nicaragua in 2005". J. Clin. Microbiol. 45 (3): 990–7. doi:10.1128/JCM.01992-06. PMID 17229854. PMC 1829148. http://jcm.asm.org/cgi/content/full/45/3/990.
- ↑ Linhares AC, Pinheiro FP, Freitas RB, Gabbay YB, Shirley JA, Beards GM (1981). "An outbreak of rotavirus diarrhea among a non-immune, isolated South American Indian community". Am. J. Epidemiol. 113 (6): 703–10. PMID 6263087.
- ↑ Hung T, Chen GM, Wang CG, et al. (1984). "Waterborne outbreak of rotavirus diarrhea in adults in China caused by a novel rotavirus". Lancet 1 (8387): 1139–42. PMID 6144874.
- ↑ Fang ZY, Ye Q, Ho MS, et al. (1989). "Investigation of an outbreak of adult diarrhea rotavirus in China". J. Infect. Dis. 160 (6): 948–53. PMID 2555422.
- ↑ Kelkar SD, Zade JK (2004). "Group B rotaviruses similar to strain CAL-1, have been circulating in Western India since 1993". Epidemiol. Infect. 132 (4): 745–9. doi:10.1017/S0950268804002171. PMID 15310177.
- ↑ Ahmed MU, Kobayashi N, Wakuda M, Sanekata T, Taniguchi K, Kader A, Naik TN, Ishino M, Alam MM, Kojima K, Mise K, Sumi A (2004). "Genetic analysis of group B human rotaviruses detected in Bangladesh in 2000 and 2001". J. Med. Virol. 72 (1): 149–55. doi:10.1002/jmv.10546. PMID 14635024.
- ↑ Penaranda ME, Ho MS, Fang ZY, et al. (1 October 1989). "Seroepidemiology of adult diarrhea rotavirus in China, 1977 to 1987" (PDF). J. Clin. Microbiol. 27 (10): 2180–3. PMID 2479654. PMC 266989. http://jcm.asm.org/cgi/reprint/27/10/2180.
- ↑ Kuzuya M, Fujii R, Hamano M, Nishijima M, Ogura H (2007). "Detection and molecular characterization of human group C rotaviruses in Okayama Prefecture, Japan, between 1986 and 2005". J. Med. Virol. 79 (8): 1219–28. doi:10.1002/jmv.20910. PMID 17596825.
- ↑ Brown DW, Campbell L, Tomkins DS, Hambling MH (1989). "School outbreak of gastroenteritis due to atypical rotavirus". Lancet 2 (8665): 737–8. doi:10.1016/S0140-6736(89)90794-0. PMID 2570978.
- ↑ Bernstein DI (March 2009). "Rotavirus overview". Pediatr. Infect. Dis. J. 28 (3 Suppl): S50–3. doi:10.1097/INF.0b013e3181967bee. PMID 19252423. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=00006454-200903001-00002. Retrieved 2009-03-15.
- ↑ O'Ryan M (2007). "Rotarix (RIX4414): an oral human rotavirus vaccine". Expert review of vaccines 6 (1): 11–9. doi:10.1586/14760584.6.1.11. PMID 17280473.
- ↑ Matson DO (2006). "The pentavalent rotavirus vaccine, RotaTeq". Seminars in paediatric infectious diseases 17 (4): 195–9. doi:10.1053/j.spid.2006.08.005. PMID 17055370.
- ↑ Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U (November 2009). "Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines". The Journal of Infectious Diseases 200 Suppl 1: S1–8. doi:10.1086/605061. PMID 19817589. http://www.journals.uchicago.edu/doi/abs/10.1086/605061?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov. Retrieved 2010-05-23.
- ↑ Accelerating access to rotavirus vaccines PATH, access date July 22, 2008
- ↑ Global use of rotavirus vaccines recommended, World Health Organization, Media centre, News, " . . . accelerated and integrated approach to combat rotavirus diarrhoea and pneumonia, the two biggest vaccine-preventable diseases . . . ," June 5, 2009.
- ↑ WHO Rotavirus vaccine.
- ↑ Ashley CR, Caul EO, Clarke SK, Corner BD, Dunn S (1978). "Rotavirus infections of apes". Lancet 2 (8087): 477. doi:10.1016/S0140-6736(78)91485-X. PMID 79844.
- ↑ Wani SA, Bhat MA, Ishaq SM, Ashrafi MA (2004). "Determination of bovine rotavirus G genotypes in Kashmir, India". Rev. – Off. Int. Epizoot. 23 (3): 931–6. PMID 15861888.
- ↑ Saif LJ (1999). "Enteric viral infections of pigs and strategies for induction of mucosal immunity". Advances in veterinary medicine 41: 429–46. doi:10.1016/S0065-3519(99)80033-0. PMID 9890034.
- ↑ Pérez-Cano FJ, Castell M, Castellote C, Franch A (2007). "Characterization of Clinical and Immune Response in a Rotavirus Diarrhea Model in Suckling Lewis Rats". Pediatr Res 62 (6): 658. doi:10.1203/PDR.0b013e318159a273. PMID 17957154.
- ↑ Enriquez C, Nwachuku N, Gerba CP (2001). "Direct exposure to animal enteric pathogens". Reviews on environmental health 16 (2): 117–31. PMID 11512628.
- ↑ Feng N, Franco MA, Greenberg HB (1997). "Murine model of rotavirus infection". Adv. Exp. Med. Biol. 412: 233–40. PMID 9192019.
- ↑ Hardy ME, Woode GN, Xu ZC, et al. (May 1991). "Analysis of serotypes and electropherotypes of equine rotaviruses isolated in the United States". J. Clin. Microbiol. 29 (5): 889–93. PMID 1647407. PMC 269902. http://jcm.asm.org/cgi/reprint/29/5/889?view=long&pmid=1647407.
- ↑ Thouless ME, DiGiacomo RF, Deeb BJ, Howard H (1 May 1988). "Pathogenicity of rotavirus in rabbits" (PDF). J. Clin. Microbiol. 26 (5): 943–7. PMID 2838507. PMC 266491. http://jcm.asm.org/cgi/reprint/26/5/943.
- ↑ Guy JS (1 August 1998). "Virus infections of the gastrointestinal tract of poultry" (PDF). Poult. Sci. 77 (8): 1166–75. PMID 9706084. http://ps.fass.org/cgi/reprint/77/8/1166.
- ↑ Müller H, Johne R (2007). "Rotaviruses: diversity and zoonotic potential—a brief review". Berl. Munch. Tierarztl. Wochenschr. 120 (3–4): 108–12. PMID 17416132.
- ↑ Cook N, Bridger J, Kendall K, Gomara MI, El-Attar L, Gray J (2004). "The zoonotic potential of rotavirus". J. Infect. 48 (4): 289–302. doi:10.1016/j.jinf.2004.01.018. PMID 15066329.
- ↑ Beards GM, Brown DW (1988). "The antigenic diversity of rotaviruses: Significance to epidemiology and vaccine strategies". Eur. J. Epidemiol. 4 (1): 1–11. doi:10.1007/BF00152685. PMID 2833405.
- ↑ Santos N, Hoshino Y (2005). "Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine". Rev. Med. Virol. 15 (1): 29–56. doi:10.1002/rmv.448. PMID 15484186.
- ↑ Desselberger U, Wolleswinkel-van den Bosch J, Mrukowicz J, Rodrigo C, Giaquinto C, Vesikari T (2006). "Rotavirus types in Europe and their significance for vaccination". Pediatr. Infect. Dis. J. 25 (1 Suppl.): S30–41. doi:10.1097/01.inf.0000197707.70835.f3. PMID 16397427. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=00006454-200601001-00005.
- ↑ Chan WK, Penaranda ME, Crawford SE, Estes MK (1986). "Two glycoproteins are produced from the rotavirus neutralization gene". Virology 151 (2): 243–52. doi:10.1016/0042-6822(86)90046-2. PMID 3010552.
- ↑ 103.0 103.1 Pesavento JB, Crawford SE, Estes MK, Prasad BV (2006). "Rotavirus proteins: structure and assembly". Curr. Top. Microbiol. Immunol. 309: 189–219. doi:10.1007/3-540-30773-7_7. PMID 16913048.
- ↑ Prasad BV, Chiu W (1994). "Structure of rotavirus". Curr. Top. Microbiol. Immunol. 185: 9–29. PMID 8050286.
- ↑ Patton JT (1995). "Structure and function of the rotavirus RNA-binding proteins" (PDF). J. Gen. Virol. 76 (Pt 11): 2633–44. doi:10.1099/0022-1317-76-11-2633. PMID 7595370. http://vir.sgmjournals.org/cgi/reprint/76/11/2633.
- ↑ Patton JT (2001). "Rotavirus RNA replication and gene expression". Novartis Found. Symp. 238: 64–77; discussion 77–81. doi:10.1002/0470846534.ch5. PMID 11444036.
- ↑ Vásquez-del Carpió R, Morales JL, Barro M, Ricardo A, Spencer E (2006). "Bioinformatic prediction of polymerase elements in the rotavirus VP1 protein". Biol. Res. 39 (4): 649–59. doi:/S0716-97602006000500008 (inactive 2009-12-20). PMID 17657346. http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0716-97602006000500008&tlng=en&lng=en&nrm=iso.
- ↑ Arnoldi F, Campagna M, Eichwald C, Desselberger U, Burrone OR (2007). "Interaction of rotavirus polymerase VP1 with nonstructural protein NSP5 is stronger than that with NSP2". J. Virol. 81 (5): 2128–37. doi:10.1128/JVI.01494-06. PMID 17182692. PMC 1865955. http://jvi.asm.org/cgi/content/full/81/5/2128.
- ↑ Fresco LD, Buratowski S (1994). "Active site of the mRNA-capping enzyme guanylyltransferase from Saccharomyces cerevisiae: similarity to the nucleotidyl attachment motif of DNA and RNA ligases" (PDF). Proc. Natl. Acad. Sci. U.S.A. 91 (14): 6624–8. doi:10.1073/pnas.91.14.6624. PMID 8022828. PMC 44255. http://www.pnas.org/content/91/14/6624.full.pdf+html.
- ↑ Gardet A, Breton M, Fontanges P, Trugnan G, Chwetzoff S (2006). "Rotavirus spike protein VP4 binds to and remodels actin bundles of the epithelial brush border into actin bodies". J. Virol. 80 (8): 3947–56. doi:10.1128/JVI.80.8.3947-3956.2006. PMID 16571811. PMC 1440440. http://jvi.asm.org/cgi/content/full/80/8/3947.
- ↑ Arias CF, Isa P, Guerrero CA, Méndez E, Zárate S, López T, Espinosa R, Romero P, López S (2002). "Molecular biology of rotavirus cell entry". Arch. Med. Res. 33 (4): 356–61. doi:10.1016/S0188-4409(02)00374-0. PMID 12234525.
- ↑ Konno T, Suzuki H, Kitaoka S, Sato T, Fukuhara N, Yoshie O, Fukudome K, Numazaki Y (1993). "Proteolytic enhancement of human rotavirus infectivity". Clin. Infect. Dis. 16 Suppl 2: S92–7. PMID 8384014.
- ↑ Hoshino Y, Jones RW, Kapikian AZ (2002). "Characterization of neutralization specificities of outer capsid spike protein VP4 of selected murine, lapine, and human rotavirus strains". Virology 299 (1): 64–71. doi:10.1006/viro.2002.1474. PMID 12167342.
- ↑ Hua J, Mansell EA, Patton JT (1993). "Comparative analysis of the rotavirus NS53 gene: conservation of basic and cysteine-rich regions in the protein and possible stem-loop structures in the RNA". Virology 196 (1): 372–8. doi:10.1006/viro.1993.1492. PMID 8395125.
- ↑ Kattoura MD, Chen X, Patton JT (1994). "The rotavirus RNA-binding protein NS35 (NSP2) forms 10S multimers and interacts with the viral RNA polymerase". Virology 202 (2): 803–13. doi:10.1006/viro.1994.1402. PMID 8030243.
- ↑ Taraporewala ZF, Patton JT (2004). "Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae". Virus Res. 101 (1): 57–66. doi:10.1016/j.virusres.2003.12.006. PMID 15010217.
- ↑ Poncet D, Aponte C, Cohen J (1 June 1993). "Rotavirus protein NSP3 (NS34) is bound to the 3' end consensus sequence of viral mRNAs in infected cells" (PDF). J. Virol. 67 (6): 3159–65. PMID 8388495. PMC 237654. http://jvi.asm.org/cgi/reprint/67/6/3159.
- ↑ Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP (1997). "The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production". Proc. Natl. Acad. Sci. U.S.A. 94 (8): 3960–5. doi:10.1073/pnas.94.8.3960. PMID 9108087. PMC 20550. http://www.pnas.org/content/94/8/3960.full.
- ↑ Afrikanova I, Miozzo MC, Giambiagi S, Burrone O (1996). "Phosphorylation generates different forms of rotavirus NSP5". J. Gen. Virol. 77 ( Pt 9): 2059–65. doi:10.1099/0022-1317-77-9-2059. PMID 8811003. http://vir.sgmjournals.org/cgi/reprint/77/9/2059.
- ↑ Rainsford EW, McCrae MA (2007). "Characterization of the NSP6 protein product of rotavirus gene 11". Virus Res. 130 (1–2): 193–201. doi:10.1016/j.virusres.2007.06.011. PMID 17658646.
- ↑ Mohan KV, Atreya CD (2001). "Nucleotide sequence analysis of rotavirus gene 11 from two tissue culture-adapted ATCC strains, RRV and Wa". Virus Genes 23 (3): 321–9. doi:10.1023/A:1012577407824. PMID 11778700.
- ↑ Desselberger U. Rotavirus: basic facts. In Rotaviruses Methods and Protocols. Ed. Gray, J. and Desselberger U. Humana Press, 2000, pp. 1–8. ISBN 0-89603-736-3
- ↑ Patton JT. Rotavirus RNA replication and gene expression. In Novartis Foundation. Gastroenteritis Viruses, Humana Press, 2001, pp. 64–81. ISBN 0-471-49663-4
- ↑ Claude M. Fauquet; J. Maniloff; Desselberger, U. (2005). Virus taxonomy: classification and nomenclature of viruses: 8th report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier/Academic Press. pp. 489. ISBN 0-12-249951-4.
- ↑ Greenberg HB, Estes MK (May 2009). "Rotaviruses: from pathogenesis to vaccination". Gastroenterology 136 (6): 1939–51. doi:10.1053/j.gastro.2009.02.076. PMID 19457420.
- ↑ Greenberg HB, Clark HF, Offit PA (1994). "Rotavirus pathology and pathophysiology". Curr. Top. Microbiol. Immunol. 185: 255–83. PMID 8050281.
- ↑ Jayaram H, Estes MK, Prasad BV (2004). "Emerging themes in rotavirus cell entry, genome organization, transcription and replication". Virus Res. 101 (1): 67–81. doi:10.1016/j.virusres.2003.12.007. PMID 15010218.
- ↑ Patton JT, Vasquez-Del Carpio R, Spencer E (2004). "Replication and transcription of the rotavirus genome". Curr. Pharm. Des. 10 (30): 3769–77. doi:10.2174/1381612043382620. PMID 15579070.
External links
- WHO Rotavirus web page
- CDC About Rotavirus
- Viralzone: Rotavirus
- Rotavirus resource library
- DefeatDD.org
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||