Metformin
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
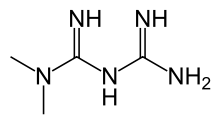
| N,N-dimethylimidodicarbonimidic diamide | |
| Identifiers | |
| CAS number | 657-24-9 |
| ATC code | A10BA02 A10BD02 (with sulfonylureas) A10BD03 (with rosiglitazone) A10BD05 (with pioglitazone) A10BD07 (with sitagliptin) A10BD08 (with vildagliptin) |
| PubChem | CID 4091 |
| DrugBank | APRD01099 |
| Chemical data | |
| Formula | C4H11N5 |
| Mol. mass | 129.164 g/mol (free) 165.63 g/mol (HCl) |
| Synonyms | 1,1-dimethylbiguanide |
| Pharmacokinetic data | |
| Bioavailability | 50 to 60% under fasting conditions |
| Metabolism | None |
| Half-life | 6.2 hours |
| Excretion | Active renal tubular excretion by OCT2 |
| Therapeutic considerations | |
| Licence data | US FDA:link |
| Pregnancy cat. | C(AU) B(US) |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Oral |
| |
|
Metformin (INN) (pronounced /mɛtˈfɔrmɪn/; originally sold as Glucophage) is an oral anti-diabetic drug in the biguanide class. It is the first-line drug of choice for the treatment of type 2 diabetes, particularly in overweight and obese people and those with normal kidney function.[1][2][3] Evidence is also mounting for its efficacy in gestational diabetes, although safety concerns still preclude its widespread use in this setting. It is also used in the treatment of polycystic ovary syndrome and has been investigated for other diseases where insulin resistance may be an important factor.
When prescribed appropriately, metformin causes few adverse effects—the most common is gastrointestinal upset—and, unlike many other anti-diabetic drugs, does not cause hypoglycemia if used alone. Lactic acidosis (a buildup of lactate in the blood) can be a serious concern in overdose and when it is prescribed to people with contraindications, but otherwise, there is no significant risk. Metformin helps reduce LDL cholesterol and triglyceride levels and is not associated with weight gain, and is the only anti-diabetic drug that has been conclusively shown to prevent the cardiovascular complications of diabetes. As of 2009[update], metformin is one of only two oral anti-diabetics in the World Health Organization Model List of Essential Medicines (the other being glibenclamide).[4]
First synthesized and found to reduce blood sugar in the 1920s, metformin was forgotten for the next two decades as research shifted to insulin and other anti-diabetic drugs. Interest in metformin was rekindled in the late 1940s after several reports that it could reduce blood sugar levels in people, and in 1957, French physician Jean Sterne published the first clinical trial of metformin as a treatment for diabetes. It was introduced to the United Kingdom in 1958, Canada in 1972, and the United States in 1995. Metformin is now believed to be the most widely prescribed anti-diabetic drug in the world; in the United States alone, more than 42 million prescriptions were filled in 2009 for its generic formulations.[5][6]
Contents |
History
The biguanide class of anti-diabetic drugs, which also includes the withdrawn agents phenformin and buformin, originates from the French lilac (Galega officinalis), a plant used in folk medicine for several centuries.[7]
Metformin was first described in the scientific literature in 1922, by Emil Werner and James Bell, as a product in the synthesis of N,N-dimethylguanidine.[8] In 1929, Slotta and Tschesche discovered its sugar-lowering action in rabbits, noting that it was the most potent of the biguanide analogs they studied.[9] This result was completely forgotten as other guanidine analogs, such as the synthalins, took over, and were themselves soon overshadowed by insulin.[10]
Interest in metformin, however, picked up at the end of the 1940s. In 1950, metformin, unlike some other similar compounds, was found not to decrease blood pressure and heart rate in animals.[11] That same year, a prominent Philippine physician, Eusebio Y. Garcia,[12] used metformin (he named it Fluamine) to treat influenza; he noted that the drug "lowered the blood sugar to minimum physiological limit" in treated patients and was non-toxic. Garcia also believed metformin to have bacteriostatic, antiviral, antimalarial, antipyretic and analgesic actions.[13] In a series of articles in 1954, Polish pharmacologist Janusz Supniewski[14] was unable to confirm most of these effects, including lowered blood sugar; he did, however, observe some antiviral effects in humans.[15][16]
While training at the Hôpital de la Pitié, French diabetologist Jean Sterne studied the antihyperglycemic properties of galegine, an alkaloid isolated from Galega officinalis, which is structurally related to metformin and had seen brief use as an anti-diabetic before the synthalins were developed.[5] Later, working at Laboratoires Aron in Paris, he was prompted by Garcia's report to re-investigate the blood sugar lowering activity of metformin and several biguanide analogs. Sterne was the first to try metformin on humans for the treatment of diabetes; he coined the name "Glucophage" (glucose eater) for the drug and published his results in 1957.[5][10]
Metformin became available in the British National Formulary in 1958. It was sold in the UK by a small Aron subsidiary called Rona. [17]
Broad interest in metformin was not rekindled until the withdrawal of the other biguanides in the 1970s. Metformin was approved in Canada in 1972,[18] but did not receive approval by the U.S. Food and Drug Administration (FDA) for type 2 diabetes until 1994.[19] Produced under license by Bristol-Myers Squibb, Glucophage was the first branded formulation of metformin to be marketed in the United States, beginning on March 3, 1995.[20] Generic formulations are now available in several countries, and metformin is believed to have become the most widely prescribed anti-diabetic drug in the world.[5]
Treatment of diabetes
The main use for metformin is in the treatment of diabetes mellitus type 2, especially in overweight people. In this group, over 10 years of treatment, metformin reduced diabetes complications and overall mortality by about 30% when compared with insulin and sulfonylureas (glibenclamide and chlorpropamide) and by about 40% when compared with the group only given dietary advice.[21] This difference held in the patients who were followed for 5–10 years after the study.[22] In addition, metformin had no effect on body weight: over the 10-year treatment period, the metformin group gained about 1 kg, the same as the dietary advice group, while the sulfonylureas group gained 3 kg, and the insulin group, 6 kg.[21][23] As metformin affords a similar level of blood sugar control to insulin and sulfonylureas, it appears to decrease mortality primarily through decreasing heart attacks, strokes and other cardiovascular complications.
Unlike the other most-commonly prescribed class of oral diabetes drugs, the sulfonylureas, metformin does not induce hypoglycemia when taken alone and used at recommended dosages.[24][25] Hypoglycemia during intense exercise has been documented, but is extremely rare.[26] It is also not associated with weight gain, and modestly reduces LDL and triglyceride levels.[24][25]
Several epidemiological and case-controlled studies found that diabetics using metformin may have lower cancer risk in comparison to those using other sugar-lowering medications. The causes of this phenomenon are unclear, and the results require confirmation in controlled studies.[27]
Off-label use
Metformin is also being used increasingly in polycystic ovary syndrome (PCOS),[28] non-alcoholic fatty liver disease (NAFLD)[29] and premature puberty,[30] three other diseases that feature insulin resistance; these indications are still[update] considered experimental. The benefit of metformin in NAFLD has not been extensively studied and may be only temporary;[31] although some randomized controlled trials have found significant improvement with its use, the evidence is still insufficient.[32][33]
Prediabetes
Metformin treatment of people at risk for type 2 diabetes may decrease their chances of developing the disease, although intensive physical exercise and dieting work significantly better for this purpose. In a large U.S. study known as the Diabetes Prevention Program, participants were divided into groups and given either placebo, metformin, or lifestyle intervention, and followed for an average of three years. The intensive program of lifestyle modifications included a 16-lesson training on dieting and exercise followed by monthly individualized sessions with the goals to decrease the body weight by 7% and engage in a physical activity for at least 150 minutes per week. The incidence of diabetes was 58% lower in the lifestyle group and 31% lower in those given metformin. Among younger people with a higher body mass index, lifestyle modification was no more effective than metformin, and for older individuals with a lower body mass index, metformin was no better than placebo in preventing diabetes.[34] After ten years, the incidence of diabetes was 34% lower in the group of participants given diet and exercise and 18% lower in those given metformin.[35] It is unclear whether metformin slowed down the progression of pre-diabetes to diabetes (true preventive effect), or the decrease of diabetes in the treated population was simply due to its glucose-lowering action (treatment effect).[36]
Polycystic ovary syndrome
Antidiabetic therapy has been proposed as a treatment for polycystic ovary syndrome (PCOS), a condition frequently associated with insulin resistance, since the late 1980s.[37] The use of metformin in PCOS was first reported in 1994, in a small study conducted at the University of the Andes, Venezuela.[38][39] The United Kingdom's National Institute for Health and Clinical Excellence recommended in 2004 that women with PCOS and a body mass index above 25 be given metformin for anovulation and infertility when other therapy has failed to produce results.[40] However, two large clinical studies completed in 2006–2007 returned mostly negative results, with metformin being no better than placebo and metformin-clomifene combination no better than clomifene alone.[41][42] Reflecting this, subsequent reviews noted that large randomized control trials have in general not shown the promise suggested by the early small studies. U.K. and international clinical practice guidelines do not recommend metformin as a first-line treatment[43] or do not recommend it at all, except for women with glucose intolerance.[44] The guidelines suggest clomiphene as the first medication option and emphasize lifestyle modification independently from the drug treatment.
In a dissenting opinion, a systematic review of four head-to-head comparative trials of metformin and clomifene found them equally effective for infertility.[45] A BMJ editorial noted that four positive studies of metformin were in patients who did not respond to clomifene, while the population in the negative studies was drug-naive or uncontrolled for the previous treatment. The editorial suggested that metformin should be used as a second-line drug if clomifene treatment fails.[46] Another review recommended metformin unreservedly as a first-line treatment option because it has positive effects not only on anovulation but also on insulin resistance, hirsutism, and obesity often associated with PCOS.[47] A large Cochrane Collaboration review of 27 randomized clinical trials found that metformin improves ovulation and pregnancy rates, particularly when combined with clomifene, but is not associated with any increase in the number of live births.[48]
The design of the negative trials may be one of the explanations for the contradictory results. For example, using live birth rate instead of pregnancy as the end point may have biased some trials against metformin, which works slower than clomifene.[49] Another explanation may be different efficacy of metformin in different populations of patients. The negative trials contained large percent of obese and previously untreated patients whose response to metformin may be weaker.[49]
Gestational diabetes
Several observational studies and randomized controlled trials have found that metformin is as effective and safe as insulin for the management of gestational diabetes,[50][51][52] and a small case-control study has suggested that the children of women given metformin instead of insulin may be healthier in the neonatal period.[53] Nonetheless, several concerns have been raised regarding studies published thus far, and evidence on the long-term safety of metformin for both mother and child is still lacking.[54]
Investigational findings
A large case-control study conducted at M.D. Anderson Cancer Center has suggested that metformin may protect against pancreatic cancer. The risk of pancreatic cancer in study participants who took metformin was found to be 62% lower than in participants who had never taken it, whereas participants who had used insulin or secretagogues (such as the sulfonylureas) were found to have a 5-fold and 2.5-fold higher risk of pancreatic cancer, respectively, compared to participants that had been treated with neither.[55] The study had several limitations, however, and the reason for this risk reduction is still unclear.[55] Observational studies conducted by the University of Dundee have shown a decrease of 25–37% in cancer cases in diabetics taking metformin.[56][57]
A single randomized controlled trial suggested that metformin may reduce weight gain in patients taking atypical antipsychotics, particularly when combined with lifestyle interventions (education, dieting, and exercise).[58]
Formulations
Metformin is sold under several trade names, including Glucophage XR, Riomet, Fortamet, Glumetza, Obimet, Dianben, Diabex, and Diaformin.
Metformin IR (immediate release) is available in 500 mg, 850 mg, and 1000 mg tablets, all now generic in the US.
Metformin SR (slow release) or XR (extended release) was introduced in 2004, in 500 mg and 750 mg strengths, mainly to counteract the most common gastrointestinal side effects, as well as to increase patient compliance by reducing pill burden. No difference in effectiveness exists between the two preparations.
Combinations with other drugs
Metformin is often prescribed to type 2 diabetes patients in combination with other drugs. Several are available as fixed-dose combinations, also with the purpose of reducing pill burden and making administration simpler and more convenient.[59]
As of 2009, the most popular brand-name combination was metformin with rosiglitazone, sold as Avandamet by GlaxoSmithKline since 2002.[60][61] Rosiglitazone actively makes cells more sensitive to insulin, complementing the action of the metformin. In 2005, all current stock of Avandamet was seized by the FDA and removed from the market, after inspections showed the factory where it was produced was violating good manufacturing practices.[62] The drug pair continued to be prescribed separately in the absence of Avandamet, which was available again by the end of that year.
In the United States, metformin is also available in combination with pioglitazone (trade name Actoplus Met), the sulfonylureas glipizide (trade name Metaglip) and glibenclamide (known as glyburide in the United States, trade name Glucovance), the dipeptidyl peptidase-4 inhibitor sitagliptin (trade name Janumet), and the meglitinide repaglinide (PrandiMet). Generic formulations of metformin/glipizide and metformin/glibenclamide are available (the latter being more popular).[6] A generic formulation of metformin/rosiglitazone from Teva has received tentative approval from the FDA, and is expected to reach the market in early 2012.[63]
Contraindications
Metformin is contraindicated in people with any condition that could increase the risk of lactic acidosis, including kidney disorders (creatinine levels over 150 μmol/l,[64] although this is an arbitrary limit), lung disease and liver disease. According to the prescribing information, heart failure, in particular, unstable or acute congestive heart failure increases risk of lactic acidosis with metformin.[65] A 2007 systematic review of controlled trials, however, suggested that metformin is the only anti-diabetic drug not associated with any measurable harm in people with heart failure, and that it may reduce mortality in comparison with other anti-diabetic agents.[66]
It is recommended that metformin be temporarily discontinued before any radiographic study involving iodinated contrast (such as a contrast-enhanced CT scan or angiogram), as contrast dye may temporarily impair kidney function, indirectly leading to lactic acidosis by causing retention of metformin in the body.[67][68] It is recommended that metformin be resumed after two days, assuming kidney function is normal.[67][68]
Adverse effects
The most common adverse effect of metformin is gastrointestinal upset, including diarrhea, cramps, nausea, vomiting and increased flatulence; metformin is more commonly associated with gastrointestinal side effects than most other anti-diabetic drugs.[25] The most serious potential side effect of metformin use is lactic acidosis; this complication is very rare, and the vast majority of these cases seem to be related to comorbid conditions such as impaired liver or kidney function, rather than to the metformin itself.[69]
Metformin has also been reported to decrease the blood levels of thyroid-stimulating hormone in patients with hypothyroidism,[70] and, in men, lutenizing hormone and testosterone.[71][72] The clinical significance of these changes is still unknown.
Gastrointestinal
In a clinical trial of 286 subjects, 53.2% of the 141 who were given immediate-release metformin (as opposed to placebo) reported diarrhea, versus 11.7% for placebo, and 25.5% reported nausea/vomiting, versus 8.3% for those on placebo.[73]
Gastrointestinal upset can cause severe discomfort for patients; it is most common when metformin is first administered, or when the dose is increased. The discomfort can often be avoided by beginning at a low dose (1 to 1.7 grams per day) and increasing the dose gradually. Gastrointestinal upset after prolonged, steady use is less common.
Long-term use of metformin has been associated with increased homocysteine levels[74] and malabsorption of vitamin B12.[75][76] Higher doses and prolonged use are associated with increased incidence of B12 deficiency,[77] and some researchers recommend screening or prevention strategies.[78]
Lactic acidosis
The most serious potential adverse effect of biguanide use is lactic acidosis. Phenformin, another biguanide, was withdrawn from the market because of an increased risk of lactic acidosis (up to 60 cases per million patient-years). However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased by the medication so long as it is not prescribed to known high-risk groups.[79]
Lactate uptake by the liver is diminished with metformin administration because lactate is a substrate for hepatic gluconeogenesis, a process which metformin inhibits. In healthy individuals, this slight excess is simply cleared by other mechanisms (including uptake by the kidneys, when their function is unimpaired), and no significant elevation in blood levels of lactate occurs.[24] When there is impaired renal function, however, clearance of metformin (and lactate) is reduced and the drug may accumulate, leading to lactic acidosis. Because metformin decreases liver uptake of lactate, any condition which may precipitate lactic acidosis is a contraindication to its use. Common causes of increased lactic acid production include alcoholism (due to depletion of NAD+ stores), heart failure, and respiratory disease (due to inadequate oxygenation of tissues); the most common cause of impaired lactic acid excretion is kidney disease.[80]
It has also been suggested that metformin increases production of lactate in the small intestine; this could potentially contribute to lactic acidosis in patients with risk factors.[81] However, the clinical significance of this is unknown, and the risk of metformin-associated lactic acidosis is most commonly attributed to decreased hepatic uptake rather than increased intestinal production.[24][80][82]
Overdosage
A review of intentional and accidental metformin overdoses reported to poison control centers over a five-year period found that serious adverse events were rare, though elderly patients appeared to be at greater risk.[83] A similar study where cases were reported to Texas poison control centers between the years 2000 and 2006 found that ingested doses of more than 5,000 mg were more likely to involve serious medical outcomes in adults.[84] Survival following intentional overdoses with up to 63,000 mg (63 g) of metformin have been reported in the medical literature.[85] Fatalities following overdose are rare, but do occur.[86][87][88] In healthy children, unintentional doses of less than 1,700 mg are unlikely to cause any significant toxic effects.[89]
The most common symptoms following overdose appear to include vomiting, diarrhea, abdominal pain, tachycardia, drowsiness, and rarely, hypoglycemia or hyperglycemia.[84][87] The major potentially life-threatening complication of metformin overdose is lactic acidosis, which is due to lactate accumulation.[90][91] Treatment of metformin overdose is generally supportive as there is no specific antidote. Lactic acidosis is initially treated with sodium bicarbonate, although high doses are not recommended as this may increase intracellular acidosis.[88] Acidosis that does not respond to administration of sodium bicarbonate may require further management with standard hemodialysis or continuous veno-venous hemofiltration. Additionally, due to metformin’s low molecular weight and lack of plasma protein binding, these techniques also have the benefit of efficiently removing metformin from blood plasma, preventing further lactate over-production.[92][93][94]
Chemistry
The usual synthesis of metformin, originally described in 1922 and reproduced in multiple later patents and publications, involves the reaction of dimethylamine hydrochloride and 2-cyanoguanidine (dicyandiamide) with heating.[8][95]
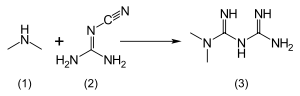
According to the procedure described in 1975 Aron patent[96] and the Pharmaceutical Manufacturing Encyclopedia,[97] equimolar amounts of dimethylamine and 2-cyanoguanidine are dissolved in toluene with cooling to make a concentrated solution, and equimolar amount of hydrogen chloride is slowly added. The mixture begins to boil on its own, and after cooling, metformin hydrochloride precipitates with 96% yield.
Pharmacokinetics
Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly.[98][99] Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin and four to eight hours with extended-release formulations.[98][99] The plasma protein binding of metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 L after a single dose). Steady state is usually reached in one or two days.[98]
Metformin is not metabolized. It is cleared from the body by tubular secretion and excreted unchanged in the urine; metformin is undetectable in blood plasma within 24 hours of a single oral dose.[98][100] The average elimination half-life in plasma is 6.2 hours.[98] Metformin is distributed to (and appears to accumulate in) red blood cells, with a much longer elimination half-life: 17.6 hours[98] (reported as ranging from 18.5 to 31.5 hours in a single-dose study of non-diabetic people).[100]
Mechanism of action
Metformin improves hyperglycemia primarily through its suppression of hepatic glucose production (hepatic gluconeogenesis).[81] The "average" person with type 2 diabetes has three times the normal rate of gluconeogenesis; metformin treatment reduces this by over one third.[101] Metformin activates AMP-activated protein kinase (AMPK), a liver enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats;[102] activation of AMPK is required for metformin's inhibitory effect on the production of glucose by liver cells.[103] Research published in 2008 further elucidated metformin's mechanism of action, showing that activation of AMPK is required for an increase in the expression of SHP, which in turn inhibits the expression of the hepatic gluconeogenic genes PEPCK and Glc-6-Pase.[104] Metformin is frequently used in research along with AICAR as an AMPK agonist. The mechanism by which biguanides increase the activity of AMPK remains uncertain; however, research suggests that metformin increases the amount of cytosolic AMP (as opposed to a change in total AMP or total AMP/ATP).[105]
In addition to suppressing hepatic glucose production, metformin increases insulin sensitivity, enhances peripheral glucose uptake, increases fatty acid oxidation,[106] and decreases absorption of glucose from the gastrointestinal tract. Increased peripheral utilization of glucose may be due to improved insulin binding to insulin receptors.[107] AMPK probably also plays a role, as metformin administration increases AMPK activity in skeletal muscle.[108] AMPK is known to cause GLUT4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake. Some metabolic actions of metformin do appear to occur by AMPK-independent mechanisms; a 2008 study found that "the metabolic actions of metformin in the heart muscle can occur independent of changes in AMPK activity and may be mediated by p38 MAPK- and PKC-dependent mechanisms."[109]
Interactions
The H2-receptor antagonist cimetidine causes an increase in the plasma concentration of metformin, by reducing clearance of metformin by the kidneys;[110] both metformin and cimetidine are cleared from the body by tubular secretion, and both, particularly the cationic (positively charged) form of cimetidine, may compete for the same transport mechanism.[98] A small double-blind, randomized study found the antibiotic cefalexin to also increase metformin concentrations by a similar mechanism;[111] theoretically, other cationic medications may produce the same effect.[98]
References
- ↑ Clinical Guidelines Task Force, International Diabetes Federation (2005). "Glucose control: oral therapy"PDF (100 KB). In: Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation, 35–8. Retrieved on November 6, 2007.
- ↑ National Collaborating Centre for Chronic Conditions. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update) [pdf]. London: Royal College of Physicians; 2008. ISBN 9781860163333. p. 86.
- ↑ American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32 Suppl 1:S13–61. doi:10.2337/dc09-S013. PMID 19118286.
- ↑ (March 2009) WHO Model List of Essential MedicinesPDF (612 KB), 16th edition, World Health Organization, p. 24. Retrieved on 19 December 2009.
- ↑ 5.0 5.1 5.2 5.3 Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115–7. doi:10.1002/pdi.606.
- ↑ 6.0 6.1 2009 Top 200 generic drugs by total prescriptionsPDF (71.7 KB). Drug Topics (June 17, 2010). Retrieved on September 2, 2010.
- ↑ Witters L. The blooming of the French lilac. J Clin Invest. 2001;108(8):1105–7. doi:10.1172/JCI14178. PMID 11602616. PMC 209536.
- ↑ 8.0 8.1 Werner E, Bell J. The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides respectively. J Chem Soc, Transactions. 1921;121:1790–5. doi:10.1039/CT9222101790.
- ↑ See Chemical Abstracts, v.23, 42772 (1929) Werner E, Bell J. Uber Biguanide. II. Die Blutzuckersenkende Wirkung der Biguanides. Berichte der Deutschen Chemischen Gesellschaft B: Abhandlungen. 1929;62:1398–1405.
- ↑ 10.0 10.1 Metformin—life begins at 50: A symposium held on the occasion of the 43rd Annual Meeting of the European Association for the Study of Diabetes, Amsterdam, The Netherlands, September 2007. The British Journal of Diabetes & Vascular Disease. 2007;7:247–252. doi:10.1177/14746514070070051001.
- ↑ Dawes GS, Mott JC. Circulatory and respiratory reflexes caused by aromatic guanidines. Br J Pharmacol Chemother. 1950;5(1):65–76. PMID 15405470.
- ↑ About Eusebio Y. Garcia, see: Carteciano J. Philippines Department of Science and Technology. Search for DOST-NRCP Dr. Eusebio Y. Garcia Award; 2005 [cited 2009-12-05].
- ↑ Quoted from Chemical Abstracts, v.45, 24828 (1951) Garcia EY. Fluamine, a new synthetic analgesic and antiflu drug. J Philippine Med Assoc. 1950;26:287–93.
- ↑ About Janusz Supniewski, see: Wołkow PP, Korbut R. Pharmacology at the Jagiellonian University in Kracow, short review of contribution to global science and cardiovascular research through 400 years of history [pdf]. J Physiol Pharmacol. 2006 [cited 2009-12-05];57 Suppl 1:119–36. PMID 16766803.
- ↑ See Chemical Abstracts, v.52, 22272 (1958) SUPNIEWSKI J, CHRUSCIEL T. [N-dimethyl-di-guanide and its biological properties.]. Arch Immunol Ther Exp (Warsz). 1954;2:1–15. Polish. PMID 13269290.
- ↑ Quoted from Chemical Abstracts, v.49, 74699 (1955) Supniewski J, Krupinska, J. [Effect of biguanide derivatives on experimental cowpox in rabbits.]. Bulletin de l'Academie Polonaise des Sciences, Classe 3: Mathematique, Astronomie, Physique, Chimie, Geologie et Geographie. 1954;2(Classe II):161–5. French.
- ↑ Hadden DR. Goat's rue - French lilac - Italian fitch - Spanish sainfoin: gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35(3):258–60. PMID 16402501.
- ↑ Lucis OJ. The status of metformin in Canada. Can Med Assoc J. 1983;128(1):24–6. PMID 6847752.
- ↑ U.S. Food and Drug Administration (December 30, 1994). "FDA Approves New Diabetes Drug". Press release. Archived from the original on 29 September 2007. http://web.archive.org/web/20070929152824/http://www.fda.gov/bbs/topics/ANSWERS/ANS00627.html. Retrieved 2007-01-06.
- ↑ GLUCOPHAGE Label and Approval History. U.S. Food and Drug Administration. Retrieved on 8 January 2007. Data available for download on FDA website.
- ↑ 21.0 21.1 Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. doi:10.1016/S0140-6736(98)07037-8. PMID 9742977.
- ↑ Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi:10.1056/NEJMoa0806470. PMID 18784090.
- ↑ Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168(19):2070–80. doi:10.1001/archinte.168.19.2070. PMID 18955635.
- ↑ 24.0 24.1 24.2 24.3 Maharani U. Chapter 27: Diabetes Mellitus & Hypoglycemia. In: Papadakis MA, McPhee SJ. CURRENT Medical Diagnosis and Treatment 2010. 49th ed. McGraw-Hill Medical; 2009. ISBN 0-07-162444-9. p. 1092–93.
- ↑ 25.0 25.1 25.2 Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–99. PMID 17638715.
- ↑ DiPiro, Joseph T.; Talbert, Robert L.; Yee, Gary C.; Matzke, Gary R.; Wells, Barbara G.; Posey, L. Michael. Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill; 2005. ISBN 0071416137.
- ↑ Chong CR, Chabner BA. Mysterious metformin. Oncologist. 2009;14(12):1178–81. doi:10.1634/theoncologist.2009-0286. PMID 20007645.
- ↑ Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327(7421):951–3. doi:10.1136/bmj.327.7421.951. PMID 14576245. PMC 259161.
- ↑ Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358(9285):893–4. doi:10.1016/S0140-6736(01)06042-1. PMID 11567710.
- ↑ Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab. 2006;91(8):2888–91. doi:10.1210/jc.2006-0336. PMID 16684823.
- ↑ Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20(1):23–28. doi:10.1111/j.1365-2036.2004.02025.x. PMID 15225167.
- ↑ Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;24(1):CD005166. doi:10.1002/14651858.CD005166.pub2. PMID 17253544.
- ↑ Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48(5):587–96. PMID 19412008.
- ↑ Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi:10.1056/NEJMoa012512. PMID 11832527.
- ↑ Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi:10.1016/S0140-6736(09)61457-4. PMID 19878986.
- ↑ Lilly M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009;55(4):363–9. PMID 19366942.
- ↑ Kidson W. Polycystic ovary syndrome: a new direction in treatment. Med J Aust. 1998;169(10):537–40. PMID 9861912.
- ↑ Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metab Clin Exp. 1994;43(5):647–54. PMID 8177055.
- ↑ Teede H. Insulin sensitizers in polycystic ovary syndrome. In: Kovács GT, Norman RW. Polycystic ovary syndrome. Cambridge, UK: Cambridge University Press; 2007. ISBN 0-521-84849-0. p. 65–81.
- ↑ National Collaborating Centre for Women’s and Children's Health. Fertility: assessment and treatment for people with fertility problems [pdf]. London: Royal College of Obstetricians and Gynaecologists; 2004. ISBN 1900364972. p. 58–9.
- ↑ Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–66. doi:10.1056/NEJMoa063971. PMID 17287476.
- ↑ Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332(7556):1485. doi:10.1136/bmj.38867.631551.55. PMID 16769748.
- ↑ Balen A. Royal College of Obstetricians and Gynaecologists. Metformin therapy for the management of infertility in women with polycystic ovary syndrome [PDF]; December 2008 [cited 2009-12-13].
- ↑ The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–77. doi:10.1093/humrep/dem426. PMID 18308833.
- ↑ Palomba S, Pasquali R, Orio F, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin. Endocrinol. (Oxf). 2009;70(2):311–21. doi:10.1111/j.1365-2265.2008.03369.x. PMID 18691273.
- ↑ Al-Inany H, Johnson N. Drugs for anovulatory infertility in polycystic ovary syndrome. BMJ. 2006;332(7556):1461–2. doi:10.1136/bmj.332.7556.1461. PMID 16793784.
- ↑ Radosh L. Drug treatments for polycystic ovary syndrome. Am Fam Physician. 2009;79(8):671–6. PMID 19405411.
- ↑ Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2009;(4):CD003053. doi:10.1002/14651858.CD003053.pub3. PMID 19821299.
- ↑ 49.0 49.1 Palomba S, Orio F, Falbo A, Russo T, Tolino A, Zullo F. Clomiphene citrate versus metformin as first-line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(9):3498–503. doi:10.1210/jc.2007-1009. PMID 17595241.
- ↑ Tertti K, Ekblad U, Vahlberg T, Rönnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud. 2008;5(2):95–101. doi:10.1900/RDS.2008.5.95. PMID 18795211. PMC 2556447.
- ↑ Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;258(19):2003–15. PMID 18463376.
- ↑ Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193–205. PMID 19104375.
- ↑ Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study. Diabet Med. 2009;26(8):798–802. PMID 19709150.
- ↑ Cheung NW. The management of gestational diabetes [pdf]. Vasc Health Risk Manag. 2009;5(1):153–64. PMID 19436673. PMC 2672462.
- ↑ 55.0 55.1 Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–8. doi:10.1053/j.gastro.2009.04.013. PMID 19375425. Lay summary: Medscape, August 18, 2009.
- ↑ Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer of 25–37% in diabetic patients. BMJ. 2005;330:1304–5. doi:10.1136/bmj.38415.708634.F7. PMID 15849206. PMC 558205.
- ↑ Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi:10.2337/dc08-2175. PMID 19564453. PMC 2732153.
- ↑ Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185–93. doi:10.1001/jama.2007.56-b. PMID 18182600.
- ↑ Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab. 2009;11(6):527–33. doi:10.1111/j.1463-1326.2008.00993.x. PMID 19175373.
- ↑ GlaxoSmithKline (October 12, 2002). "FDA Approves GlaxoSmithKline's Avandamet (rosiglitazone maleate and metformin HCl), The Latest Advancement in the Treatment of Type 2 Diabetes". Press release. http://www.docguide.com/news/content.nsf/news/8525697700573E1885256C4F0075B2B3. Retrieved 2006-12-27.
- ↑ 2009 Top 200 branded drugs by total prescriptionsPDF (96.5 KB). Drug Topics (June 17, 2010). Retrieved on September 2, 2010.
- ↑ U.S. Food and Drug Administration (March 4, 2005). "Questions and Answers about the Seizure of Paxil CR and Avandamet". Press release. Archived from the original on 14 October 2007. http://web.archive.org/web/20071014014507/http://www.fda.gov/oc/qanda/PaxilandAvandamet.html. Retrieved 2006-12-27.
- ↑ Reuters (September 27, 2007). "Teva Pharm announces settlement of generic Avandia, Avandamet, and Avandaryl litigation with GlaxoSmithKline". Press release. http://www.reuters.com/article/inPlayBriefing/idUSIN20070927170530TEVA20070927. Retrieved 2009-02-17.
- ↑ Jones G, Macklin J, Alexander W. Contraindications to the use of metformin. BMJ. 2003;326(7379):4–5. doi:10.1136/bmj.326.7379.4. PMID 12511434. PMC 1124930.
- ↑ US FDA. Glucophage Prescribing Information for the U.S. [PDF] [cited 2009-12-24].
- ↑ Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335(7618):497. doi:10.1136/bmj.39314.620174.80. PMID 17761999. PMC 1971204.
- ↑ 67.0 67.1 Weir J (March 19, 1999). Guidelines with Regard to Metformin-Induced Lactic Acidosis and X-ray Contrast Medium Agents. Royal College of Radiologists. Retrieved on 26 October 2007 through the Internet Archive.
- ↑ 68.0 68.1 Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003;76(908):513–8. doi:10.1259/bjr/26964464. PMID 12893691.
- ↑ Khurana R, Malik IS. Metformin: safety in cardiac patients. Heart. 2010;96(2):99–102. doi:10.1136/hrt.2009.173773. PMID 19564648.
- ↑ Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab. 2006;91(1):225–7. doi:10.1210/jc.2005-1210. PMID 16219720.
- ↑ Shegem NS, Nasir AM, Jbour AK, Batieha AM, El-Khateeb MS, Ajlouni KM. Effects of short term metformin administration on androgens in normal men. Saudi Med J. 2002;23(8):934–7. PMID 12235466.
- ↑ Ozata M, Oktenli C, Bingol N, Ozdemir IC. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes Res. 2001;9(11):662–7. doi:10.1038/oby.2001.90. PMID 11707532.
- ↑ Drug Facts and Comparisons 2005. St. Louis, Mo: Facts and Comparisons; 2004. ISBN 1574391933.
- ↑ Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJ, Stehouwer CD. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254(5):455–63. doi:10.1046/j.1365-2796.2003.01213.x. PMID 14535967.
- ↑ Andrès E, Noel E, Goichot B. Metformin-associated vitamin B12 deficiency. Arch Intern Med. 2002;162(19):2251–2. doi:10.1001/archinte.162.19.2251-a. PMID 12390080.
- ↑ Gilligan M. Metformin and vitamin B12 deficiency. Arch Intern Med. 2002;162(4):484–5. doi:10.1001/archinte.162.4.484. PMID 11863489.
- ↑ de Jager J, Kooy A, Lehert P, et al. (2010). "Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial". BMJ 340: c2181.
- ↑ Ting R, Szeto C, Chan M, Ma K, Chow K. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975–9. doi:10.1001/archinte.166.18.1975. PMID 17030830.
- ↑ Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163(21):2594–602. doi:10.1001/archinte.163.21.2594. PMID 14638559.
- ↑ 80.0 80.1 Shu AD, Myers Jr MG, Shoelson SE. Chapter 29: Pharmacology of the Endocrine Pancreas. In: Golan ED et al. (eds.). Principles of pharmacology: the pathophysiologic basis of drug therapy. Philadelphia: Lippincott, Williams & Wilkins; 2005. ISBN 0-7817-4678-7. p. 540–41.
- ↑ 81.0 81.1 Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update [PDF]. Ann Intern Med. 2002;137(1):25–33. PMID 12093242.
- ↑ Davis SN. Chapter 60: Insulin, Oral Hypoglycemic Agents, and the Pharmacology of the Endocrine Pancreas. In: Brunton L, Lazo J, Parker K. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. ISBN 978-0071422802.
- ↑ Spiller HA, Quadrani DA. Toxic effects from metformin exposure. Ann Pharmacother. 2004;38(5):776–80. doi:10.1345/aph.1D468. PMID 15031415.
- ↑ 84.0 84.1 Forrester MB. Adult metformin ingestions reported to Texas poison control centers, 2000-2006. Hum Exp Toxicol. 2008;27(7):575–83. doi:10.1177/0960327108090589. PMID 18829734.
- ↑ Gjedde S, Christiansen A, Pedersen SB, Rungby J. Survival following a metformin overdose of 63 g: a case report. Pharmacol Toxicol. 2003;93(2):98–9. doi:10.1034/j.1600-0773.2003.930207.x. PMID 12899672.
- ↑ Nisse P, Mathieu-Nolf M, Deveaux M, Forceville X, Combes A. A fatal case of metformin poisoning. J Toxicol Clin Toxicol. 2003;41(7):1035–6. doi:10.1081/CLT-120026533. PMID 14705855.
- ↑ 87.0 87.1 Suchard JR, Grotsky TA. Fatal metformin overdose presenting with progressive hyperglycemia. West J Emerg Med. 2008;9(3):160–4. PMID 19561734.
- ↑ 88.0 88.1 Teale KF, Devine A, Stewart H, Harper NJ. The management of metformin overdose. Anaesthesia. 1998;53(7):698–701. doi:10.1046/j.1365-2044.1998.436-az0549.x. PMID 9771180.
- ↑ Spiller HA, Weber JA, Winter ML, Klein-Schwartz W, Hofman M, Gorman SE, Stork CM, Krenzelok EP. Multicenter case series of pediatric metformin ingestion. Ann Pharmacother. 2000;34(12):1385–8. doi:10.1345/aph.10116. PMID 11144693.
- ↑ Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54(6):818–23. doi:10.1016/j.annemergmed.2009.04.023. PMID 19556031.
- ↑ Lacher M, Hermanns-Clausen M, Haeffner K, Brandis M, Pohl M. Severe metformin intoxication with lactic acidosis in an adolescent. Eur J Pediatr. 2005;164(6):362–5. doi:10.1007/s00431-005-1634-y. PMID 15729560.
- ↑ Harvey B, Hickman C, Hinson G, Ralph T, Mayer A. Severe lactic acidosis complicating metformin overdose successfully treated with high-volume venovenous hemofiltration and aggressive alkalinization. Pediatr Crit Care Med. 2005;6(5):598–601. doi:10.1097/01.PCC.0000162451.47034.4F. PMID 16148825.
- ↑ Guo PY, Storsley LJ, Finkle SN. Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin. Semin Dial. 2006;19(1):80–3. doi:10.1111/j.1525-139X.2006.00123.x. PMID 16423187.
- ↑ Barrueto F, Meggs WJ, Barchman MJ. Clearance of metformin by hemofiltration in overdose. J Toxicol Clin Toxicol. 2002;40(2):177–80. doi:10.1081/CLT-120004407. PMID 12126190.
- ↑ Shapiro SL, Parrino VA, Freedman L. Hypoglycemic Agents. I Chemical Properties of β-Phenethylbiguanide. A New Hypoglycemic Agent. J Am Chem Soc. 1959;81(9):2220–5. doi:10.1021/ja01518a052.
- ↑ Procédé de préparation de chlorhydrate de diméthylbiguanide. Patent FR 2322860. 1975. French.
- ↑ Pharmaceutical Manufacturing Encyclopedia (Sittig's Pharmaceutical Manufacturing Encyclopedia). 3rd ed. Vol. 3. Norwich, NY: William Andrew; 2007. ISBN 0-8155-1526-X. p. 2208.
- ↑ 98.0 98.1 98.2 98.3 98.4 98.5 98.6 98.7 Bristol-Myers Squibb. U.S. Food and Drug Administration. Glucophage® (metformin hydrochloride tablets) Label Information; August 27, 2008 [cited 2009-12-08].
- ↑ 99.0 99.1 Heller JB. Metformin overdose in dogs and cats. Veterinary Medicine. 2007;(April):231–233.
- ↑ 100.0 100.1 Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29(3):279–83. PMID 12909816.
- ↑ Hundal R, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi S, Schumann W, Petersen K, Landau B, Shulman G. Mechanism by which metformin reduces glucose production in type 2 diabetes [PDF]. Diabetes. 2000;49(12):2063–9. doi:10.2337/diabetes.49.12.2063. PMID 11118008.
- ↑ Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–41. doi:10.1161/01.RES.0000256090.42690.05. PMID 17307971.
- ↑ Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman M, Goodyear L, Moller D. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi:10.1172/JCI13505. PMID 11602624.
- ↑ Kim YD, Park KG, Lee YS, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57(2):306–14. doi:10.2337/db07-0381. PMID 17909097.
- ↑ Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293(1):H457–66. doi:10.1152/ajpheart.00002.2007. PMID 17369473.
- ↑ Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291(1):E182–E189. doi:10.1152/ajpendo.00272.2005. PMID 16478780.
- ↑ Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–9. doi:10.1056/NEJM199602293340906. PMID 8569826.
- ↑ Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–81. doi:10.2337/diabetes.51.7.2074. PMID 12086935.
- ↑ Saeedi R, Parsons HL, Wambolt RB, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294(6):H2497–506. doi:10.1152/ajpheart.00873.2007. PMID 18375721.
- ↑ Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;23(5):545–51. PMID 3593625.
- ↑ Jayasagar G, Krishna Kumar M, Chandrasekhar K, Madhusudan Rao C, Madhusudan Rao Y. Effect of cephalexin on the pharmacokinetics of metformin in healthy human volunteers. Drug Metabol Drug Interact. 2002;19(1):41–8. PMID 12222753.
External links
- Metformin at the Open Directory Project
- Metformin drug information from Lexi-Comp. Includes dosage information and a comprehensive list of international brand names
- U.S. National Library of Medicine: Drug Information Portal - Metformin
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||