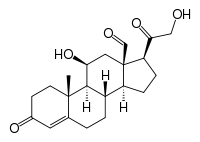
Aldosterone
| Aldosterone | |
|---|---|
 |
|
|
11β,21-dihydroxy-3,20-dioxopregn-4-en-18-al
|
|
| Identifiers | |
| CAS number | 52-39-1 |
| PubChem | 5839 |
| ChemSpider | 5633 |
| MeSH | Aldosterone |
|
SMILES
O=C(CO)[C@@H]4[C@@]3(C=O)C[C@H](O)[C@@H]2[C@@]1(/C(=C\C(=O)CC1)CC[C@H]2[C@@H]3CC4)C
|
|
|
InChI
InChI=1/C21H28O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h8,11,14-17,19,22,25H,2-7,9-10H2,1H3/t14-,15-,16+,17-,19+,20-,21+/m0/s1
Key: PQSUYGKTWSAVDQ-ZVIOFETBBV |
|
| Properties | |
| Molecular formula | C21H28O5 |
| Molar mass | 360.44 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Aldosterone is a hormone that increases the reabsorption of sodium and water and the release (secretion) of potassium in the kidneys. This increases blood volume and, therefore, increases blood pressure. Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives. One example is spironolactone, which lowers blood pressure by blocking the aldosterone receptor. Aldosterone is part of the renin-angiotensin system. A measurement of aldosterone in blood may be termed a plasma aldosterone concentration (PAC), which may be compared to plasma renin activity (PRA) as a PAC/PRA ratio.
Aldosterone is a steroid hormone (mineralocorticoid family) produced by the outer-section (zona glomerulosa) of the adrenal cortex in the adrenal gland, and acts on the distal tubules and collecting ducts of the kidney to cause the conservation of sodium, secretion of potassium, increased water retention, and increased blood pressure. The overall effect of aldosterone is to increase reabsorption of ions and water in the kidney.
Its activity is reduced in Addison's disease and increased in Conn syndrome.
It was first isolated by Simpson and Tait in 1953.[1]
Contents |
Synthesis
The corticosteroids are synthesized from cholesterol within the adrenal cortex. Most steroidogenic reactions are catalysed by enzymes of the cytochrome P450 family. They are located within the mitochondria and require adrenodoxin as a cofactor (except 21-hydroxylase and 17α-hydroxylase).
Aldosterone and corticosterone share the first part of their biosynthetic pathway. The last part is mediated either by the aldosterone synthase (for aldosterone) or by the 11β-hydroxylase (for corticosterone). These enzymes are nearly identical (they share 11β-hydroxylation and 18-hydroxylation functions). But aldosterone synthase is also able to perform a 18-oxidation. Moreover, aldosterone synthase is found within the zona glomerulosa at the outer edge of the adrenal cortex; 11β-hydroxylase is found in the zona fasciculata and reticularis.
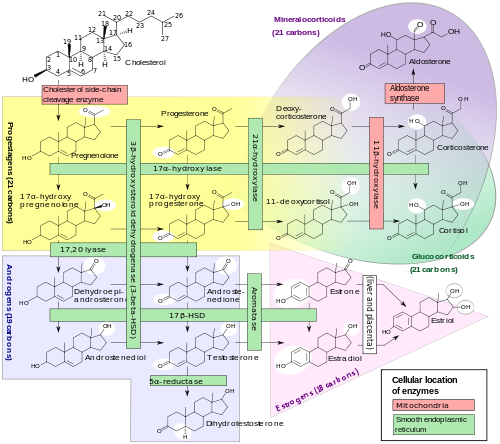
Note: aldosterone synthase is absent in other sections of the adrenal gland.
Stimulation
Aldosterone synthesis is stimulated by several factors:
- increase in the plasma concentration of Angiotensin III, a metabolite of Angiotensin II
- increase in plasma angiotensin II, ACTH, or potassium levels, which are present in proportion to plasma sodium deficiencies. (The increased potassium level works to regulate aldosterone synthesis by depolarizing the cells in the zona glomerulosa, which opens the voltage-dependent calcium channels.) The level of angiotensin II is regulated by angiotensin I, which is in turn regulated by the hormone renin. Potassium levels are the most sensitive stimulator of aldosterone.
- the ACTH stimulation test, which is sometimes used to stimulate the production of aldosterone along with cortisol to determine whether primary or secondary adrenal insufficiency is present (However, ACTH has only a minor role in regulating aldosterone production; with hypopituitarism there is no atrophy of the zona glomerulosa.)
- plasma acidosis
- the stretch receptors located in the atria of the heart. If decreased blood pressure is detected, the adrenal gland is stimulated by these stretch receptors to release aldosterone, which increases sodium reabsorption from the urine, sweat, and the gut. This causes increased osmolarity in the extracellular fluid, which will eventually return blood pressure toward normal.
- adrenoglomerulotropin, a lipid factor, obtained from pineal extracts. It selectively stimulates secretion of aldosterone [2].
The secretion of aldosterone has a diurnal rhythm.[3]
Function
Aldosterone is the primary of several endogenous members of the class of mineralocorticoids in human. Deoxycorticosterone is another important member of this class. Aldosterone tends to promote Na+ and water retention, and lower plasma K+ concentration by the following mechanisms:
- Acting on the nuclear mineralocorticoid receptors (MR) within the principal cells of the distal tubule and the collecting duct of the kidney nephron, it up regulates and activates the basolateral Na+/K+ pumps, stimulating ATP hydrolysis leading to phosphorylation of the pump and a conformational change in the pump exposes the Na+ ions to the outside. The phosphorylated form of the pump has a low affinity for Na+ ions, hence reabsorbing sodium (Na+) ions and water into the blood, and secreting potassium (K+) ions into the urine.
- Aldosterone upregulates epithelial sodium channel (ENaC) increasing apical membrane permeability for Na+.
- Cl- is reabsorbed in conjunction with sodium cations to maintain the system's electrochemical balance.
- Aldosterone stimulates uptake of K+ into cells.
- Aldosterone stimulates Na+ and water reabsorption from the gut salivary and sweat glands in exchange for K+.
- Aldosterone stimulates H+ secretion by intercalated cells in the collecting duct, regulating plasma bicarbonate (HCO3−) levels and its acid/base balance.[4]
- Aldosterone may act on the central nervous system via the posterior pituitary gland to release vasopressin (ADH), which serves to conserve water by direct actions on renal tubular reabsorption.
Aldosterone is responsible for the reabsorption of about 2% of filtered sodium in the kidneys, which is nearly equal to the entire sodium content in human blood under normal GFR (glomerular filtration rate).[5]
Aldosterone, most probably acting through mineralocorticoid receptors, may positively influence neurogenesis in the dentate gyrus. [6]
Location of receptors
Unlike neuroreceptors, classic steroid receptors are intracellular. The aldosterone mineralcorticoid receptor (MR) complex binds on the DNA to specific hormone response element, which leads to gene specific transcription.
Some of the transcribed genes are crucial for transepithelial sodium transport, including the three subunits of the epithelial sodium channel (ENaC), the Na+/K+ pumps and their regulatory proteins serum and glucocorticoid-induced kinase, and channel-inducing factor, respectively.
The mineralcorticoid receptor is stimulated by both aldosterone and cortisol, but there is a mechanism that protects the body from excess aldosterone receptor stimulation by glucocorticoids, which happen to be present at much higher concentrations than mineralcorticoids in the healthy individual. The mechanism consists of an enzyme called 11 β-hydroxysteroid dehydrogenase (11 β-HSD). This enzyme co-localizes with intracellular adrenal steroid receptors and converts cortisol (an active mineralcorticoid) into cortisone, a relatively inactive metabolite with little affinity for the MR. Licorice, which contains glycyrrhetinic acid, can inhibit 11 β-HSD and lead to a mineralcorticoid excess syndrome.
Control of aldosterone release from the adrenal cortex
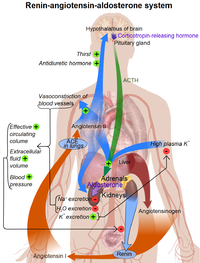
Major regulators
The role of the renin-angiotensin system
Angiotensin is involved in regulating aldosterone and is the core regulation.[8] Angiotensin II acts synergistically with potassium, and the potassium feedback is virtually inoperative when no angiotensin II is present.[9] A small portion of the regulation resulting from angiotensin II must take place indirectly from decreased blood flow through the liver due to constriction of capillaries.[10] When the blood flow decreases so does the destruction of aldosterone by liver enzymes.
The plasma concentration of potassium
The amount of aldosterone secreted is a direct function of the serum potassium [11][12] as probably determined by sensors in the carotid artery.[13][14]
ACTH
ACTH, a pituitary peptide, also has some stimulating effect on aldosterone probably by stimulating the formation of deoxycorticosterone, a precursor of aldosterone.[15] Aldosterone is increased by blood loss,[16] pregnancy,[17] and possibly by other circumstances such as physical exertion, endotoxin shock, and burns.[18][19]
Miscellaneous regulators
The role of sympathetic nerves
The aldosterone production is also affected to one extent or another by nervous control, which integrates the inverse of carotid artery pressure,[13] pain, posture,[17] and probably emotion (anxiety, fear, and hostility) [20] (including surgical stress).[21] Anxiety increases aldosterone,[20] which must have evolved because of the time delay involved in migration of aldosterone into the cell nucleus.[22] Thus, there is an advantage to an animal's anticipating a future need from interaction with a predator, since too high a serum content of potassium has very adverse effects on nervous transmission.
The role of baroreceptors
Pressure in the carotid artery decreases aldosterone [13]
The role of the juxtaglomerular apparatus
Through RAAS
The plasma concentration of sodium
Aldosterone is a function of the inverse of the sodium intake as sensed via osmotic pressure.[23] The slope of the response of aldosterone to serum potassium is almost independent of sodium intake.[24] Aldosterone is much increased at low sodium intakes, but the rate of increase of plasma aldosterone as potassium rises in the serum is not much lower at high sodium intakes than it is at low. Thus, the potassium is strongly regulated at all sodium intakes by aldosterone when the supply of potassium is adequate, which it usually is in primitive diets.
Aldosterone feedback
Feedback by aldosterone concentration itself is of a non-morphological character (that is other than changes in the cells' number or structure) and is poor so the electrolyte feedbacks predominate short term.[18]
Additional images
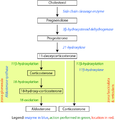 Corticosteroid biosynthetic pathway in rat |
 Corticosterone |
See also
- Aldosterone antagonist
- ACTH stimulation test
- Hyperaldosteronism
- Hypoaldosteronism
References
- ↑ Williams JS, Williams GH (June 2003). "50th anniversary of aldosterone". J Clin Endocrinol Metab. 88 (6): 2364–72. doi:10.1210/jc.2003-030490. PMID 12788829. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=12788829.
- ↑ Farrell G (May 1960). "Adrenoglomerulotropin". Circulation 21: 1009–15. PMID 13821632. http://circ.ahajournals.org/cgi/content/abstract/21/5/1009.
- ↑ Hurwitz S, Cohen RJ, Williams GH (April 2004). "Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest". J App Physiol 96 (4): 1406–14. doi:10.1152/japplphysiol.00611.2003. PMID 14660513. http://jap.physiology.org/cgi/content/full/96/4/1406.
- ↑ Rector, Floyd C.; Brenner, Barry M. (2004). Brenner & Rector's the kidney. Philadelphia: Saunders. ISBN 0-7216-0164-2. OCLC 51838812.
- ↑ Sherwood, Lauralee (2001). Human physiology: from cells to systems. Pacific Grove, CA: Brooks/Cole. ISBN 0-534-56826-2. OCLC 43702042.
- ↑ Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czéh B (August 2002). "The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat". Brain Res. 947 (2): 290–3. doi:10.1016/S0006-8993(02)03042-1. PMID 12176172. http://linkinghub.elsevier.com/retrieve/pii/S0006899302030421.
- ↑ Page 866-867 (Integration of Salt and Water Balance) and 1059 (The Adrenal Gland) in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 1300. ISBN 1-4160-2328-3.
- ↑ Williams GH, Dluhy RG (November 1972). "Aldosterone biosynthesis. Interrelationship of regulatory factors". Am J Med 53 (5): 595–605. doi:10.1016/0002-9343(72)90156-8. PMID 4342886. http://linkinghub.elsevier.com/retrieve/pii/0002-9343(72)90156-8.
- ↑ Pratt JH (September 1982). "Role of angiotensin II in potassium-mediated stimulation of aldosterone secretion in the dog". J Clin Invest. 70 (3): 667–72. doi:10.1172/JCI110661. PMID 6286729.
- ↑ Messerli FH, Nowaczynski W, Honda M, et al. (February 1977). "Effects of angiotensin II on steroid metabolism and hepatic blood flow in man". Circulation Research 40 (2): 204–7. PMID 844145.
- ↑ Bauer JH, Gauntner WC (March 1979). "Effect of potassium chloride on plasma renin activity and plasma aldosterone during sodium restriction in normal man". Kidney Int. 15 (3): 286–93. doi:10.1038/ki.1979.37. PMID 513492.
- ↑ Linas SL, Peterson LN, Anderson RJ, Aisenbrey GA, Simon FR, Berl T (June 1979). "Mechanism of renal potassium conservation in the rat". Kidney Int. 15 (6): 601–11. doi:10.1038/ki.1979.79. PMID 222934.
- ↑ 13.0 13.1 13.2 Gann DS Mills IH Bartter 1960 On the hemodynamic parameter mediating increase in aldosterone secretion in the dog. Fed. Proceedings 19; 605-610.
- ↑ Gann DS, Cruz JF, Casper AG, Bartter FC (May 1962). "Mechanism by which potassium increases aldosterone secretion in the dog". Am J Physiol. 202: 991–6. PMID 13896654. http://ajplegacy.physiology.org/cgi/pmidlookup?view=long&pmid=13896654.
- ↑ Brown RD, Strott CA, Liddle GW (June 1972). "Site of stimulation of aldosterone biosynthesis by angiotensin and potassium". J Clin Invest. 51 (6): 1413–8. doi:10.1172/JCI106937. PMID 4336939.
- ↑ Ruch TC Fulton JF 1960 Medical Physiology and Biophysics. W.B. Saunders and Co., Phijl & London. On p1099.
- ↑ 17.0 17.1 Farrell G (October 1958). "Regulation of aldosterone secretion". Physiological Reviews 38 (4): 709–28. PMID 13590935. http://physrev.physiology.org/cgi/pmidlookup?view=long&pmid=13590935.
- ↑ 18.0 18.1 Vecsei, Pál; Gláz, Edith (1971). Aldosterone. New York: Pergamon Press. ISBN 0-08-013368-1. OCLC 186705.
- ↑ Farrell GL, Rauschkolb EW (November 1956). "Evidence for diencephalic regulation of aldosterone secretion". Endocrinology 59 (5): 526–31. doi:10.1210/endo-59-5-526. PMID 13375573. on 529
- ↑ 20.0 20.1 Venning EH, DyrenfurthY I, Beck JC (August 1957). "Effect of anxiety upon aldosterone excretion in man". J Clin Endocrinol Metab. 17 (8): 1005–8. doi:10.1210/jcem-17-8-1005. PMID 13449153.
- ↑ Elman R, Shatz BA, Keating RE, Weichselbaum TE (July 1952). "Intracellular and extracellular potassium deficits in surgical patients". Annals of surgery 136 (1): 111–31. doi:10.1097/00000658-195208000-00013. PMID 14934025.
- ↑ Sharp GUG Leaf A 1966 in; Recent Progress in Hormone Research.(Pincus G, ed.
- ↑ Schneider EG, Radke KJ, Ulderich DA, Taylor RE (April 1985). "Effect of osmolality on aldosterone secretion". Endocrinology 116 (4): 1621–6. doi:10.1210/endo-116-4-1621. PMID 3971930.
- ↑ Dluhy RG, Axelrod L, Underwood RH, Williams GH (August 1972). "Studies of the control of plasma aldosterone concentration in normal man. II. Effect of dietary potassium and acute potassium infusion". J Clin Invest. 51 (8): 1950–7. doi:10.1172/JCI107001. PMID 5054456.
|
|||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||