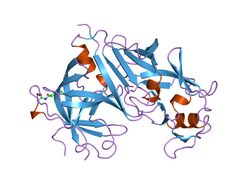
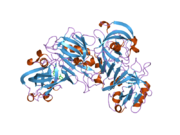
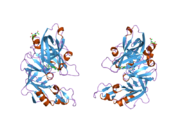
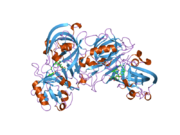
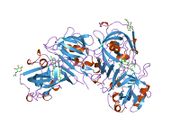
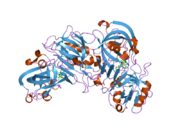
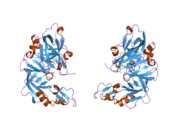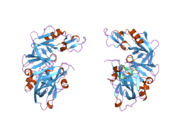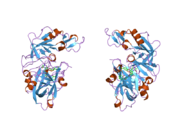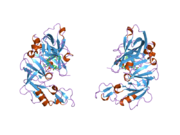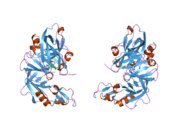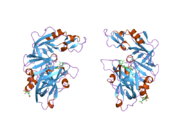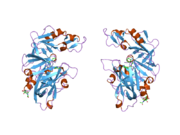Renin
Renin (pronounced /ˈriːnɨn/ REE-nin), also known as angiotensinogenase is an enzyme that participates in the body's renin-angiotensin system (RAS) that mediates extracellular volume (i.e., that of the blood plasma, lymph and interstitial fluid), and arterial vasoconstriction. Thus, it regulates the body's mean arterial blood pressure.
Contents |
Discovery
Renin was discovered, characterized, and named in 1898 by Robert Tigerstedt, Professor of Physiology at the Karolinska Institute in Stockholm.[1][2]
Biochemistry and Physiology
Structure
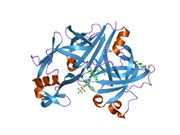
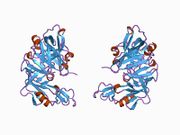
The primary structure of renin precursor consists of 406 amino acids with a pre- and a pro-segment carrying 20 and 46 amino acids, respectively. Mature renin contains 340 amino acids and has a mass of 37 kDa.[3]
Secretion
The peptide hormone is secreted by the kidney from specialized cells called granular cells of the juxtaglomerular apparatus via 3 responses:
- 1) A decrease in arterial blood pressure (that could be related to a decrease in blood volume) as detected by baroreceptors (pressure-sensitive cells). This is the most causal link between blood pressure and renin secretion (the other two methods operate via longer pathways).
- 2) A decrease in sodium chloride levels in the ultra-filtrate of the nephron. This flow is measured by the macula densa of the juxtaglomerular apparatus.
- 3) Sympathetic nervous system activity, which also controls blood pressure, acting through the beta1 adrenergic receptors.
Human Renin is secreted by at least 2 cellular pathways: a constitutive pathway for the secretion of prorenin and a regulated pathway for the secretion of mature renin.[4]
The Renin-Angiotensin-Aldosterone Axis / Renin-Angiotensin System (RAS)
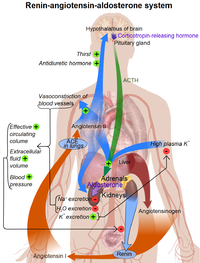
- Mechanism of action of Renin:
The enzyme circulates in the blood stream and breaks down (hydrolyzes) angiotensinogen secreted from the liver into the peptide angiotensin I.
- Rest of the RAS:
Angiotensin I is further cleaved in the lungs by endothelial-bound angiotensin converting enzyme (ACE) into angiotensin II, the most vasoactive peptide.[6][7] Angiotensin II is a potent constrictor of all blood vessels. It acts on the musculature and, therefore, raises the resistance posed by these arteries to the heart. The heart, trying to overcome this increase in its 'load', works more vigorously, causing the blood pressure to rise. Angiotensin II also acts on the adrenal glands and releases Aldosterone, which stimulates the epithelial cells in the distal tubule and collecting ducts of the kidneys to increase re-absorption of salt and water, leading to raised blood volume and raised blood pressure. The RAS also acts on the CNS to increase water intake by stimulating thirst, as well as conserving blood volume, by reducing urinary loss through the secretion of Vasopressin from the posterior pituitary gland.
The normal concentration of renin in adult human plasma is 1.98-24.6 ng/L in the upright position.[8]
Function
Renin activates the renin-angiotensin system by cleaving angiotensinogen, produced by the liver, to yield angiotensin I, which is further converted into angiotensin II by ACE, the angiotensin-converting enzyme primarily within the capillaries of the lungs. Angiotensin II then constricts blood vessels, increases the secretion of ADH and aldosterone, and stimulates the hypothalamus to activate the thirst reflex, each leading to an increase in blood pressure.
Renin is secreted from kidney cells (of the afferent arterioles), which are activated via signaling (the release of prostaglandins) from the macula densa, which respond to the rate of fluid flow through the distal tubule, by decreases in renal perfusion pressure (through stretch receptors in the vascular wall), and by nervous stimulation, mainly through beta-1 receptor activation. A drop in the rate of flow past the macula densa implies a drop in renal filtration pressure. Renin's primary function is therefore to eventually cause an increase in blood pressure, leading to restoration of perfusion pressure in the kidneys.
Renin can bind to ATP6AP2, which results in a fourfold increase in the conversion of angiotensinogen to angiotensin I over that shown by soluble renin. In addition, renin binding results in phosphorylation of serine and tyrosine residues of ATP6AP2.[9]
The level of renin mRNA appears to be modulated by the binding of HADHB, HuR and CP1 to a regulatory region in the 3' UTR.[10]
Genetics
The gene for renin, REN, spans 12 kb of DNA and contains 8 introns.[11] It produces several mRNA that encode different REN isoforms.
Clinical applications
An over-active renin-angiotension system leads to vasoconstriction and retention of sodium and water. These effects lead to hypertension. Therefore, renin inhibitors can be used for the treatment of hypertension.[12][13] This is measured by the plasma renin activity (PRA).
In current medical practice, the renin-angiotensin-aldosterone-System's overactivity (and resultant hypertension) is more commonly reduced using either ACE inhibitors (such as ramipril and perindopril) or angiotensin II receptor blockers (ARBs, such as losartan, irbesartan or candesartan) rather than a direct oral renin inhibitor. ACE inhibitors or ARBs are also part of the standard treatment after a heart attack.
The differential diagnosis of kidney cancer in a young patient with hypertension includes juxtaglomerular cell tumor (reninoma), Wilms' tumor, and renal cell carcinoma, all of which may produce renin.[14]
See also
- Renin inhibitor
- Angiotensin-converting enzyme
- plasma renin activity.
- Renin stability regulatory element (REN-SRE)
References
- ↑ Phillips, MI.; Schmidt-Ott, KM. (Dec 1999). "The Discovery of Renin 100 Years Ago.". News Physiol Sci 14: 271–274. PMID 11390864.
- ↑ Tigerstedt, R.; Bergman, P. (1898). "Niere und Kreislauf". Scandinavian Archives of Physiology 8: 223–271.
- ↑ Imai T, Miyazaki H, Hirose S, et al. (December 1983). "Cloning and sequence analysis of cDNA for human renin precursor". Proc. Natl. Acad. Sci. U.S.A. 80 (24): 7405–9. doi:10.1073/pnas.80.24.7405. PMID 6324167. PMC 389959. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=6324167.
- ↑ Pratt RE, Flynn JA, Hobart PM, Paul M, Dzau VJ (March 1988). "Different secretory pathways of renin from mouse cells transfected with the human renin gene". J. Biol. Chem. 263 (7): 3137–41. PMID 2893797. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=2893797.
- ↑ Page 866-867 (Integration of Salt and Water Balance) and 1059 (The Adrenal Gland) in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 1300. ISBN 1-4160-2328-3.
- ↑ Fujino T, Nakagawa N, Yuhki K, et al. (September 2004). "Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP". J. Clin. Invest. 114 (6): 805–12. doi:10.1172/JCI21382. PMID 15372104. PMC 516260. http://www.jci.org/cgi/content/full/114/6/805?ijkey=e3335f0a9a7b40386d49e7172910ea6345c9342a.
- ↑ Brenner & Rector's The Kidney, 7th ed., Saunders, 2004. pp.2118-2119.Full Text with MDConsult subscription
- ↑ Hamilton Regional Laboratory Medicine Program - Laboratory Reference Centre Manual. [1]
- ↑ Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD (June 2002). "Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin". J. Clin. Invest. 109 (11): 1417–27. doi:10.1172/JCI14276. PMID 12045255.
- ↑ Adams DJ, Beveridge DJ, van der Weyden L, Mangs H, Leedman PJ, Morris BJ (2003). "HADHB, HuR, and CP1 bind to the distal 3'-untranslated region of human renin mRNA and differentially modulate renin expression". J. Biol. Chem. 278 (45): 44894–903. doi:10.1074/jbc.M307782200. PMID 12933794.
- ↑ Hobart PM, Fogliano M, O'Connor BA, Schaefer IM, Chirgwin JM (August 1984). "Human renin gene: structure and sequence analysis". Proc. Natl. Acad. Sci. U.S.A. 81 (16): 5026–30. doi:10.1073/pnas.81.16.5026. PMID 6089171. PMC 391630. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=6089171.
- ↑ Presentation on Direct Renin Inhibitors as Antihypertensive Drugs
- ↑ Ram CV (September 2009). "Direct inhibition of renin: a physiological approach to treat hypertension and cardiovascular disease". Future Cardiol 5 (5): 453–65. doi:10.2217/fca.09.31. PMID 19715410.
- ↑ Méndez GP, Klock C, Nosé V (December 2008). "Juxtaglomerular Cell Tumor of the Kidney: Case Report and Differential Diagnosis With Emphasis on Pathologic and Cytopathologic Features". Int. J. Surg. Pathol.. doi:10.1177/1066896908329413. PMID 19098017.
External links
- The MEROPS online database for peptidases and their inhibitors: A01.007
- MeSH Renin
- renin at eMedicine Dictionary
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||