Progesterone
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| pregn-4-ene-3,20-dione | |
| Identifiers | |
| CAS number | 57-83-0 |
| ATC code | G03DA04 |
| PubChem | CID 5994 |
| IUPHAR ligand | 2377 |
| DrugBank | DB00396 |
| Chemical data | |
| Formula | C21H30O2 |
| Mol. mass | 314.46 |
| Synonyms | 4-pregnene-3,20-dione |
| Physical data | |
| Melt. point | 126 °C (259 °F) |
| Spec. rot | [α]D |
| Pharmacokinetic data | |
| Bioavailability | prolonged absorption, half-life approx 25-50 hours |
| Protein binding | 96%-99% |
| Metabolism | hepatic to pregnanediols and pregnanolones |
| Half-life | 34.8-55.13 hours |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. | B (USA) |
| Legal status | ? |
| Routes | oral, implant |
| |
|
Progesterone also known as P4 (pregn-4-ene-3,20-dione) is a C-21 steroid hormone involved in the female menstrual cycle, pregnancy (supports gestation) and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestogens, and is the major naturally occurring human progestogen.
Progesterone is commonly manufactured from the yam family, Dioscorea. Dioscorea produces large amounts of a steroid called diosgenin, which can be converted into progesterone in the laboratory.
Contents |
Chemistry
Progesterone was independently discovered by four research groups.[1][2][3][4]
Willard Myron Allen co-discovered progesterone with his anatomy professor George Washington Corner at the University of Rochester Medical School in 1933. Allen first determined its melting point, molecular weight, and partial molecular structure. He also gave it the name Progesterone derived from Progestational Steroidal ketone.[5]
Like other steroids, progesterone consists of four interconnected cyclic hydrocarbons. Progesterone contains ketone and oxygenated functional groups, as well as two methyl branches. Like all steroid hormones, it is hydrophobic.
Sources
Animal
Progesterone is produced in the ovaries (to be specific, after ovulation in the corpus luteum), the adrenal glands (near the kidney), and, during pregnancy, in the placenta. Progesterone is also stored in adipose (fat) tissue.
In humans, increasing amounts of progesterone are produced during pregnancy:
- At first, the source is the corpus luteum that has been "rescued" by the presence of human chorionic gonadotropins (hCG) from the conceptus.
- However, after the 8th week, production of progesterone shifts to the placenta. The placenta utilizes maternal cholesterol as the initial substrate, and most of the produced progesterone enters the maternal circulation, but some is picked up by the fetal circulation and used as substrate for fetal corticosteroids. At term the placenta produces about 250 mg progesterone per day.
- An additional source of progesterone is milk products. They contain much progesterone because on dairy farms cows are milked during pregnancy, when the progesterone content of the milk is high. After consumption of milk products the level of bioavailable progesterone goes up.[6]
Plants
In at least one plant, Juglans regia, progesterone has been detected.[7] In addition, progesterone-like steroids are found in Dioscorea mexicana. Dioscorea mexicana is a plant that is part of the yam family native to Mexico.[8] It contains a steroid called diosgenin that is taken from the plant and is converted into progesterone.[9] Diosgenin and progesterone are found in other Dioscorea species as well.
Another plant that contains substances readily convertible to progesterone is Dioscorea pseudojaponica native to Taiwan. Research has shown that the Taiwanese yam contains saponins — steroids that can be converted to diosgenin and thence to progesterone.[10]
Many other Dioscorea species of the yam family contain steroidal substances from which progesterone can be produced. Among the more notable of these are Dioscorea villosa and Dioscorea polygonoides. One study showed that the Dioscorea villosa contains 3.5% diosgenin.[11] Dioscorea polygonoides has been found to contain 2.64% diosgenin as shown by gas chromatography-mass spectrometry.[12] Many of the Dioscorea species that originate from the yam family grow in countries that have tropical and subtropical climates.[13]
Synthesis
Biosynthesis
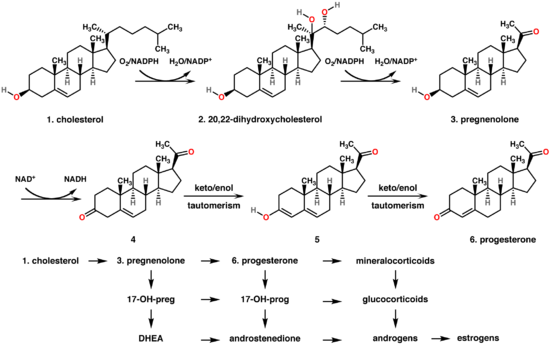
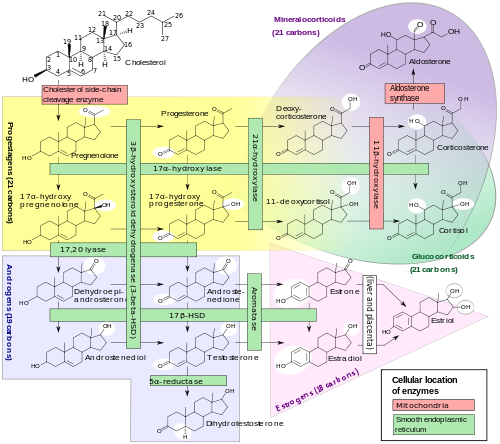
Bottom: Progesterone is important for aldosterone (mineralocorticoid) synthesis, as 17-hydroxyprogesterone is for cortisol (glucocorticoid), and androstenedione for sex steroids.
In mammals, progesterone (6), like all other steroid hormones, is synthesized from pregnenolone (3), which in turn is derived from cholesterol (1) (see the upper half of the figure to the right).
Cholesterol (1) undergoes double oxidation to produce 20,22-dihydroxycholesterol (2). This vicinal diol is then further oxidized with loss of the side chain starting at position C-22 to produce pregnenolone (3). This reaction is catalyzed by cytochrome P450scc. The conversion of pregnenolone to progesterone takes place in two steps. First, the 3-hydroxyl group is oxidized to a keto group (4) and second, the double bond is moved to C-4, from C-5 through a keto/enol tautomerization reaction.[14] This reaction is catalyzed by 3beta-hydroxysteroid dehydrogenase/delta(5)-delta(4)isomerase.
Progesterone in turn (see lower half of figure to the right) is the precursor of the mineralocorticoid aldosterone, and after conversion to 17-hydroxyprogesterone (another natural progestogen) of cortisol and androstenedione. Androstenedione can be converted to testosterone, estrone and estradiol.
Pregenolone and progesterone can also be synthesized by yeast.[15]
Laboratory
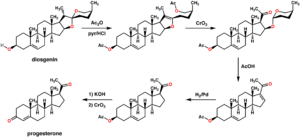
An economical semisynthesis of progesterone from the plant steroid diosgenin isolated from yams was developed by Russell Marker in 1940 for the Parke-Davis pharmaceutical company (see figure to the right).[16] This synthesis is known as the Marker degradation. Additional semisyntheses of progesterone have also been reported starting from a variety of steroids. For the example, cortisone can be simultaneously deoxygenated at the C-17 and C-21 position by treatment with iodotrimethylsilane in chloroform to produce 11-keto-progesterone (ketogestin), which in turn can be reduced at position-11 to yield progesterone.[17]
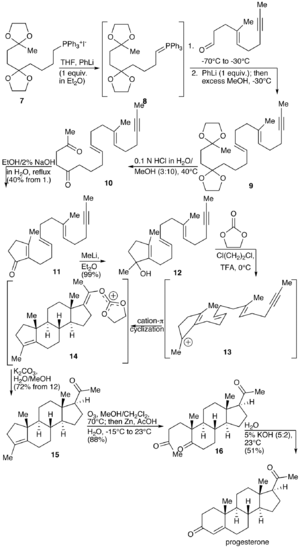
A total synthesis of progesterone was reported in 1971 by W.S. Johnson (see figure to the right).[18] The synthesis begins with reacting the phosphonium salt 7 with phenyl lithium to produce the phosphonium ylide 8. The ylide 8 is reacted with an aldehyde to produce the alkene 9. The ketal protecting groups of 9 are hydrolyzed to produce the diketone 10, which in turn is cyclized to from the cyclopentenone 11. The ketone of 11 is reacted with methyl lithium to yield the tertiary alcohol 12, which in turn is treated with acid to produce the tertiary cation 13. The key step of the synthesis is the π-cation cyclization of 13 in which the B-, C-, and D-rings of the steroid are simultaneously formed to produce 14. This step resembles the cationic cyclization reaction used in the biosynthesis of steroids and hence is referred to as biomimetic. In the next step the enol orthoester is hydrolyzed to produce the ketone 15. The cyclopentene A-ring is then opened by oxidizing with ozone to produce 16. Finally, the diketone 17 undergoes an intramolecular aldol condensation by treating with aqueous potassium hydroxide to produce progesterone.[18]
Levels
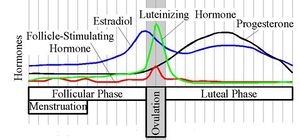
Progesterone levels (black line) during the menstrual cycle
|
In women, progesterone levels are relatively low during the preovulatory phase of the menstrual cycle, rise after ovulation, and are elevated during the luteal phase. Progesterone levels tend to be < 2 ng/ml prior to ovulation, and > 5 ng/ml after ovulation. If pregnancy occurs, progesterone levels are initially maintained at luteal levels. With the onset of the luteal-placental shift in progesterone support of the pregnancy, levels start to rise further and may reach 100-200 ng/ml at term. Whether a decrease in progesterone levels is critical for the initiation of labor has been argued and may be species-specific. After delivery of the placenta and during lactation, progesterone levels are very low.
Progesterone levels are relatively low in children and postmenopausal women.[19] Adult males have levels similar to those in women during the follicular phase of the menstrual cycle.
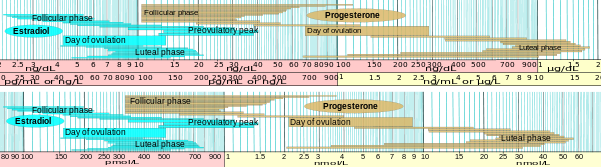
Reference ranges for estradiol and progesterone in the menstrual cycle, expressed in mass and molar concentration. Because of slightly different molar mass, the relative concentrations differ somewhat. The scale is logarithmic.
|
Effects
Progesterone exerts its primary action through the intracellular progesterone receptor although a distinct, membrane bound progesterone receptor has also been postulated.[20][21] In addition, progesterone is a highly potent antagonist of the mineralocorticoid receptor (MR, the receptor for aldosterone and other mineralocorticosteroids). It prevents MR activation by binding to this receptor with an affinity exceeding even those of aldosterone and other corticosteroids such as cortisol and corticosterone.[22]
Progesterone has a number of physiological effects that are amplified in the presence of estrogen. Estrogen through estrogen receptors upregulates the expression of progesterone receptors.[23] Also, elevated levels of progesterone potently reduce the sodium-retaining activity of aldosterone, resulting in natriuresis and a reduction in extracellular fluid volume. Progesterone withdrawal, on the other hand, is associated with a temporary increase in sodium retention (reduced natriuresis, with an increase in extracellular fluid volume) due to the compensatory increase in aldosterone production, which combats the blockade of the mineralocorticoid receptor by the previously elevated level of progesterone.[24]
Reproductive system
Progesterone has key effects via non-genomic signalling on human sperm as they migrate through the female tract before fertilisation occurs, though the receptor(s) as yet remain unidentified.[25] Detailed characterisation of the events occurring in sperm in response to progesterone has elucidated certain events including intracellular calcium transients and maintained changes,[26] slow calcium oscillations,[27] now thought to possibly regulate motility.[28] Interestingly progesterone has also been shown to demonstrate effects on octopus spermatozoa.[29]
Progesterone is sometimes called the "hormone of pregnancy",[30] and it has many roles relating to the development of the fetus:
- Progesterone converts the endometrium to its secretory stage to prepare the uterus for implantation. At the same time progesterone affects the vaginal epithelium and cervical mucus, making it thick and impenetrable to sperm. If pregnancy does not occur, progesterone levels will decrease, leading, in the human, to menstruation. Normal menstrual bleeding is progesterone-withdrawal bleeding.
- During implantation and gestation, progesterone appears to decrease the maternal immune response to allow for the acceptance of the pregnancy.
- Progesterone decreases contractility of the uterine smooth muscle.[30]
- In addition progesterone inhibits lactation during pregnancy. The fall in progesterone levels following delivery is one of the triggers for milk production.
- A drop in progesterone levels is possibly one step that facilitates the onset of labor.
The fetus metabolizes placental progesterone in the production of adrenal steroids.
Nervous system
Progesterone, like pregnenolone and dehydroepiandrosterone, belongs to the group of neurosteroids. It can be synthesized within the central nervous system and also serves as a precursor to another major neurosteroid, allopregnanolone.
Neurosteroids affect synaptic functioning, are neuroprotective, and affect myelination.[31] They are investigated for their potential to improve memory and cognitive ability. Progesterone affects regulation of apoptotic genes.
Its effect as a neurosteroid works predominantly through the GSK-3 beta pathway, as an inhibitor. (Other GSK-3 beta inhibitors include bipolar mood stabilizers, lithium and valproic acid.)
Other systems
- It raises epidermal growth factor-1 levels, a factor often used to induce proliferation, and used to sustain cultures, of stem cells.
- It increases core temperature (thermogenic function) during ovulation.[32]
- It reduces spasm and relaxes smooth muscle. Bronchi are widened and mucus regulated. (Progesterone receptors are widely present in submucosal tissue.)
- It acts as an antiinflammatory agent and regulates the immune response.
- It reduces gall-bladder activity.[33]
- It normalizes blood clotting and vascular tone, zinc and copper levels, cell oxygen levels, and use of fat stores for energy.
- It may affect gum health, increasing risk of gingivitis (gum inflammation) and tooth decay.
- It appears to prevent endometrial cancer (involving the uterine lining) by regulating the effects of estrogen.
Adverse effects
Pill form of progesterone (actually a synthetic version such as Progestogen) taken at 400 mg as cited by the following patent can cause increased fluid retention, which may result in epilepsy, migraine, asthma, cardiac or renal dysfunction. Blood clots that can result in strokes and heart attacks, which may lead to death or long-term disability, may develop; pulmonary embolus or breast cancer can also develop as a result of progesterone therapy. Progesterone is associated with an increased risk of thrombotic disorders such as thrombophlebitis, cerebrovascular disorders, pulmonary embolism, and retinal thrombosis.[34]
Common adverse effects include cramps, abdominal pain, skeletal pain, perineal pain, headache, arthralgia, constipation, dyspareunia, nocturia, diarrhea, nausea, vomiting, breast enlargement, joint pain, flatulence, hot flushes, decreased libido, thirst, increased appetite, nervousness, drowsiness, excessive urination at night. Psychiatric effects including depression, mood swings, emotional instability, aggression, abnormal crying, insomnia, forgetfulness, sleep disorders.[34]
Less frequent adverse effects that may occur include allergy, anemia, bloating, fatigue, tremor, urticaria, pain, conjunctivitis, dizziness, vomiting, myalgia, back pain, breast pain, genital itching, genital yeast infection, upper respiratory tract infection, cystitis, dysuria, asthenia, xerophthalmia, syncope, dysmenorrhea, premenstrual tension, gastritis, urinary tract infection, vaginal discharge, pharyngitis, sweating, hyperventilation, vaginal dryness, dyspnea, fever, edema, flu-like symptoms, dry mouth, rhinitis, leg pain, skin discoloration, skin disorders, seborrhea, sinusitis, acne. [34]
Current research suggests that progesterone plays an important role in the signaling of insulin release and pancreatic function, and may affect the susceptibility to diabetes[35]. It has been shown that women with high levels of progesterone during pregnancy are more likely to develop glucose abnormalities.[36]
Medical applications
The use of progesterone and its analogues have many medical applications, both to address acute situations and to address the long-term decline of natural progesterone levels. Because of the poor bioavailability of progesterone when taken orally, many synthetic progestins have been designed with improved oral bioavailability.[37] Progesteone was approved by the United States Food and Drug Administration as vaginal gel on July 31, 1997,[38] an oral capsule on May 14, 1998[39] in an injection form on April 25, 2001[40] and as a vaginal insert on June 21, 2007.[41]
Bioavailability
The route of administration impacts the affect of the drug. Give orally, progesterone has a wide person-to-person variability in absorption and bioavailability while synthetic progestins are rapidly absorbed with a longer half-life than progesterone and maintain stable levels in the blood.[42]
Progesterone does not dissolve in water and is poorly absorbed when taken orally unless micronized in oil. Products are often sold as capsules containing micronised progesterone in oil. Progesterone can also be administered through vaginal or rectal suppositories or pessaries, transdermally through a gel or cream,[43] or via injection (though the latter has a short half-life requiring daily administration).
"Natural progesterone" products derived from yams, do not require a prescription but there is no evidence the human body can convert its active ingredient (diosgenin, the plant steroid that is chemically converted to produce progesterone industrially[16]) into progesterone.[44][45]
Specific uses
- Progesterone is used to support pregnancy in Assisted Reproductive Technology (ART) cycles such as In-vitro Fertilization (IVF). While daily intramuscular injections of progesterone-in-oil (PIO) have been the standard route of administration, PIO injections are not FDA-approved for use in pregnancy. A recent meta-analysis showed that the intravaginal route with an appropriate dose and dosing frequency is equivalent to daily intramuscular injections.[46] In addition, a recent case-matched study comparing vaginal progesterone with PIO injections showed that live birth rates were nearly identical with both methods.[47]
- Progesterone is used to control anovulatory bleeding. It is also used to prepare uterine lining in infertility therapy and to support early pregnancy. Patients with recurrent pregnancy loss due to inadequate progesterone production may receive progesterone.
- Progesterone is being investigated as potentially beneficial in treating multiple sclerosis, since the characteristic deterioration of nerve myelin insulation halts during pregnancy, when progesterone levels are raised; deterioration commences again when the levels drop.
- Vaginally dosed progesterone is being investigated as potentially beneficial in preventing preterm birth in women at risk for preterm birth. The initial study by Fonseca suggested that vaginal progesterone could prevent preterm birth in women with a history of preterm birth.[48]
A subsequent and larger study showed that vaginal progesterone was no better than placebo in preventing recurrent preterm birth in women with a history of a previous preterm birth,[49] but a planned secondary analysis of the data in this trial showed that women with a short cervix at baseline in the trial had benefit in two ways: a reduction in births less than 32 weeks and a reduction in both the frequency and the time their babies were in intensive care.[50] In another trial, vaginal progesterone was shown to be better than placebo in reducing preterm birth prior to 34 weeks in women with an extremely short cervix at baseline.[51] An editorial by Roberto Romero discusses the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment.[52]
- Progesterone is used in hormone therapy for male to female transsexuals and other women with intersex conditions - especially when synthetic progestins have been ineffective or caused side-effects - since normal breast tissue cannot develop except in the presence of both progestogen and estrogen. Mammary glandular tissue is otherwise fibrotic, the breast shape conical and the areola immature. Progesterone can correct those even after years of inadequate hormonal treatment. Research usually cited against such value was conducted using Provera, a synthetic progestin. Progesterone also has a role in skin elasticity and bone strength, in respiration, in nerve tissue and in female sexuality, and the presence of progesterone receptors in certain muscle and fat tissue may hint at a role in sexually-dimorphic proportions of those. [53]
- Progesterone receptor antagonists, or selective progesterone receptor modulators (SPRM)s, such as RU-486 (Mifepristone), can be used to prevent conception or induce medical abortions.
Note that methods of hormonal contraception do not contain progesterone but a progestin.
Progesterone may affect male behavior.[54]
Progesterone is starting to be used in the treatment of the skin condition hidradenitis suppurativa.
Aging
Since most progesterone in males is created during testicular production of testosterone, and most in females by the ovaries, the shutting down (whether by natural or chemical means), or removal, of those inevitably causes a considerable reduction in progesterone levels. Previous concentration upon the role of progestagens (progesterone and molecules with similar effects) in female reproduction, when progesterone was simply considered a "female hormone", obscured the significance of progesterone elsewhere in both sexes.
The tendency for progesterone to have a regulatory effect, the presence of progesterone receptors in many types of body tissue, and the pattern of deterioration (or tumor formation) in many of those increasing in later years when progesterone levels have dropped, is prompting widespread research into the potential value of maintaining progesterone levels in both males and females.
Brain damage
Previous studies have shown that progesterone supports the normal development of neurons in the brain, and that the hormone has a protective effect on damaged brain tissue. It has been observed in animal models that females have reduced susceptibility to traumatic brain injury and this protective effect has been hypothesized to be caused by increased circulating levels of estrogen and progesterone in females.[55] A number of additional animal studies have confirmed that progesterone has neuroprotective effects when administered shortly after traumatic brain injury.[56] Encouraging results have also been reported in human clinical trials.[57][58]
The mechanism of progesterone protective effects may be the reduction of inflammation that follows brain trauma.[59]
See also
- Willard Myron Allen
- Endometrin
- Prometrium
- AKR1C1 - the enzyme that deactivates progesterone
References
- ↑ Allen WM (1935). "The isolation of crystalline progestin". Science 82 (2118): 89–93. doi:10.1126/science.82.2118.89. PMID 17747122.
- ↑ Butenandt A, Westphal U (1934). "Zur Isolierung und Charakterisierung des Corpusluteum-Hormons". Berichte Deutsche chemische Gesellschaft 67: 1440–1442. doi:10.1002/cber.19340670831.
- ↑ Hartmann M, Wettstein A (1934). "Ein krystallisiertes Hormon aus Corpus luteum". Helvetica Chimica Acta 17: 878–882. doi:10.1002/hlca.193401701111.
- ↑ Slotta KH, Ruschig H, Fels E (1934). "Reindarstellung der Hormone aus dem Corpusluteum". Berichte Deutsche chemische Gesellschaft 67: 1270–1273. doi:10.1002/cber.19340670729.
- ↑ Allen WM (1970). "Progesterone: how did the name originate?". South. Med. J. 63 (10): 1151–5. PMID 4922128.
- ↑ Goodson III WH, Handagama P, Moore II DH, Dairkee S (2007-12-13). "Milk products are a source of dietary progesterone". 30th Annual San Antonio Breast Cancer Symposium. pp. abstract # 2028. http://www.docguide.com/news/content.nsf/news/852571020057CCF6852573B1007803AD. Retrieved 2008-03-12.
- ↑ Pauli GF, Friesen JB, Gödecke T, Farnsworth NR, Glodny B (January 2010). "Occurrence of Progesterone and Related Animal Steroids in Two Higher Plants". J Nat Prod 73 (3): 338–45. doi:10.1021/np9007415. PMID 20108949.
- ↑ Applezweig N (May 1969). "Steroids". Chem Week 104: 57–72. PMID 12255132.
- ↑ Noguchi E, Fujiwara Y, Matsushita S, Ikeda T, Ono M, Nohara T (September 2006). "Metabolism of tomato steroidal glycosides in humans". Chem. Pharm. Bull. 54 (9): 1312–4. doi:10.1248/cpb.54.1312. PMID 16946542.
- ↑ Yang DJ, Lu TJ, Hwang LS (October 2003). "Isolation and identification of steroidal saponins in Taiwanese yam cultivar (Dioscorea pseudojaponica Yamamoto)". J. Agric. Food Chem. 51 (22): 6438–44. doi:10.1021/jf030390j. PMID 14558759.
- ↑ Hooker E (2004). "Final report of the amended safety assessment of Dioscorea Villosa (Wild Yam) root extract". Int. J. Toxicol. 23 Suppl 2: 49–54. doi:10.1080/10915810490499055. PMID 15513824.
- ↑ Niño J, Jiménez DA, Mosquera OM, Correa YM (2007). "Diosgenin quantification by HPLC in a Dioscorea polygonoides tuber collection from colombian flora". Journal of the Brazilian Chemical Society 18 (5): 1073–1076. doi:10.1590/S0103-50532007000500030.
- ↑ Myoda T, Nagai T, Nagashima T (2005). Properties of starches in yam (Dioscorea spp.) tuber. 105–114. ISBN 81-308-0003-9
- ↑ Dewick, Paul M. (2002). Medicinal natural products: a biosynthetic approach. New York: Wiley. pp. 244. ISBN 0-471-49641-3.
- ↑ Duport C, Spagnoli R, Degryse E, Pompon D (February 1998). "Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast". Nat. Biotechnol. 16 (2): 186–9. doi:10.1038/nbt0298-186. PMID 9487528.
- ↑ 16.0 16.1 16.2 Marker RE, Krueger J (1940). "Sterols. CXII. Sapogenins. XLI. The Preparation of Trillin and its Conversion to Progesterone". J. Am. Chem. Soc. 62 (12): 3349–3350. doi:10.1021/ja01869a023.
- ↑ Numazawa M, Nagaoka M, Kunitama Y (September 1986). "Regiospecific deoxygenation of the dihydroxyacetone moiety at C-17 of corticoid steroids with iodotrimethylsilane". Chem. Pharm. Bull. 34 (9): 3722–6. PMID 3815593. http://www.journalarchive.jst.go.jp/english/jnlabstract_en.php?cdjournal=cpb1958&cdvol=34&noissue=9&startpage=3722.
- ↑ 18.0 18.1 18.2 Johnson WS, Gravestock MB, McCarry BE (August 1971). "Acetylenic bond participation in biogenetic-like olefinic cyclizations. II. Synthesis of dl-progesterone". J. Am. Chem. Soc. 93 (17): 4332–4. doi:10.1021/ja00746a062. PMID 5131151.
- ↑ NIH Clinical Center (2004-08-16). "Progesterone Historical Reference Ranges". United States National Institutes of Health. http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index/CB26894E1EB28DEF85256BA5005B000E?OpenDocument. Retrieved 2008-03-12.
- ↑ Luconi M, Bonaccorsi L, Maggi M, Pecchioli P, Krausz C, Forti G, Baldi E (1998). "Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane". J. Clin. Endocrinol. Metab. 83 (3): 877–85. doi:10.1210/jc.83.3.877. PMID 9506743.
- ↑ Jang S, Yi LS (2005). "Identification of a 71 kDa protein as a putative non-genomic membrane progesterone receptor in boar spermatozoa". J. Endocrinol. 184 (2): 417–25. doi:10.1677/joe.1.05607. PMID 15684349.
- ↑ Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K (1993). "Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands". Eur J Pharmacol 247 (2): 145–54. doi:10.1016/0922-4106(93)90072-H. PMID 8282004.
- ↑ Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P (1990). "Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B". EMBO J. 9 (5): 1603–14. PMID 2328727.
- ↑ Landau RL, Bergenstal DM, Lugibihl K, Kascht ME. (1955). "The metabolic effects of progesterone in man". J Clin Endocrinol Metab 15 (10): 1194–215. doi:10.1210/jcem-15-10-1194. PMID 13263410.
- ↑ Correia JN, Conner SJ, Kirkman-Brown JC (May 2007). "Non-genomic steroid actions in human spermatozoa. "Persistent tickling from a laden environment"". Semin. Reprod. Med. 25 (3): 208–19. doi:10.1055/s-2007-973433. PMID 17447210.
- ↑ Kirkman-Brown JC, Bray C, Stewart PM, Barratt CL, Publicover SJ (June 2000). "Biphasic elevation of [Ca(2+)](i) in individual human spermatozoa exposed to progesterone". Developmental Biology 222 (2): 326–35. doi:10.1006/dbio.2000.9729. ISSN 0012-1606. PMID 10837122.
- ↑ Kirkman-Brown JC, Barratt CL, Publicover SJ (March 2004). "Slow calcium oscillations in human spermatozoa". The Biochemical Journal 378 (Pt 3): 827–32. doi:10.1042/BJ20031368. PMID 14606954.
- ↑ Harper CV, Barratt CL, Publicover SJ (October 2004). "Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating". The Journal of Biological Chemistry 279 (44): 46315–25. doi:10.1074/jbc.M401194200. PMID 15322137.
- ↑ Tosti E, Di Cosmo A, Cuomo A, Di Cristo C, Gragnaniello G (May 2001). "Progesterone induces activation in Octopus vulgaris spermatozoa". Mol. Reprod. Dev. 59 (1): 97–105. doi:10.1002/mrd.1011. PMID 11335951.
- ↑ 30.0 30.1 Bowen R (2000-08-06). "Placental Hormones". http://www.vivo.colostate.edu/hbooks/pathphys/reprod/placenta/endocrine.html. Retrieved 2008-03-12.
- ↑ Schumacher M, Guennoun R, Robert F, et al. (2004). "Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination". Growth Horm. IGF Res. 14 Suppl A: S18–33. doi:10.1016/j.ghir.2004.03.007. PMID 15135772.
- ↑ Physiology at MCG 5/5ch9/s5ch9_13
- ↑ Hould FS, Fried GM, Fazekas AG, Tremblay S, Mersereau WA (1988). "Progesterone receptors regulate gallbladder motility". J. Surg. Res. 45 (6): 505–12. doi:10.1016/0022-4804(88)90137-0. PMID 3184927.
- ↑ 34.0 34.1 34.2 Columbia Laboratories, Inc. (November 2004). "Prometrium(progesterone)" (PDF). http://www.prometrium.com/pdf/Prometrium_Patient_Information.pdf.
- ↑ Picard F, Wanatabe M, Schoonjans K, Lydon J, O'Malley BW, Auwerx J (November 2002). "Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation" (PDF). http://www.pnas.org/content/99/24/15644.full.pdf.
- ↑ Brănişteanu DD, Mathieu C (March 2003). "Progesterone in gestational diabetes mellitus: guilty or not guilty?". http://www.ncbi.nlm.nih.gov/pubmed/12591170.
- ↑ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins". Maturitas 61 (1-2): 171–80. doi:10.1016/j.maturitas.2003.09.014. PMID 19434889. http://www1.elsevier.com/homepage/sab/womenshealth/doc/journals/maturitas_si/2.pdf.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 020701". Food and Drug Administration. 2010-07-02. http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=020701&TABLE1=OB_Rx. Retrieved 2010-07-07.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 019781". Food and Drug Administration. 2010-07-02. http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=019781&TABLE1=OB_Rx. Retrieved 2010-07-07.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 075906". Food and Drug Administration. 2010-07-02. http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=075906&TABLE1=OB_Rx. Retrieved 2010-07-07.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 022057". Food and Drug Administration. 2010-07-02. http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=022057&TABLE1=OB_Rx. Retrieved 2010-07-07.
- ↑ PMID 14670641 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Lark, Susan (1999). Making the Estrogen Decision. McGraw-Hill Professional. p. 22. ISBN 0879836962, 9780879836962. http://books.google.com/?id=d3IP-dmpoNsC&pg=PA22&dq=progesterone+%22skin+cream%22+liver&cd=3#v=onepage&q=progesterone%20%22skin%20cream%22%20liver.
- ↑ Zava DT, Dollbaum CM, Blen M (1998). "Estrogen and progestin bioactivity of foods, herbs, and spices". Proc. Soc. Exp. Biol. Med. 217 (3): 369–78. PMID 9492350. http://www.cancersupportivecare.com/estrogenherbref.html#31.
- ↑ Komesaroff PA, Black CV, Cable V, Sudhir K (2001). "Effects of wild yam extract on menopausal symptoms, lipids and sex hormones in healthy menopausal women". Climacteric 4 (2): 144–50. doi:10.1080/713605087. PMID 11428178.
- ↑ Zarutskiea PW, Phillips JA (2007). "Re-analysis of vaginal progesterone as luteal phase support (LPS) in assisted reproduction (ART) cycles". Fertility and Sterility 88 (supplement 1): S113. doi:10.1016/j.fertnstert.2007.07.365.
- ↑ Khan N, Richter KS, Blake EJ, et al. Case-matched comparison of intramuscular versus vaginal progesterone for luteal phase support after in vitro fertilization and embryo transfer. Presented at: 55th Annual Meeting of the Pacific Coast Reproductive Society; April 18-22, 2007; Rancho Mirage, CA.
- ↑ da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M (2003). "Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study". Am. J. Obstet. Gynecol. 188 (2): 419–24. doi:10.1067/mob.2003.41. PMID 12592250.
- ↑ O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma-Pillay P, Porter K, How H, Schackis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW (2007). "Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial". Ultrasound Obstet Gynecol 30 (5): 687–96. doi:10.1002/uog.5158. PMID 17899572.
- ↑ DeFranco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, Soma-Pillay P, Porter K, How H, Schakis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW (2007). "Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial". Ultrasound Obstet Gynecol 30 (5): 697–705. doi:10.1002/uog.5159. PMID 17899571.
- ↑ Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH (2007). "Progesterone and the risk of preterm birth among women with a short cervix". N. Engl. J. Med. 357 (5): 462–9. doi:10.1056/NEJMoa067815. PMID 17671254.
- ↑ Romero R (2007). "Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment". Ultrasound Obstet Gynecol 30 (5): 675–86. doi:10.1002/uog.5174. PMID 17899585.
- ↑ Sriram, D (2007). Medicinal Chemistry. New Delhi: Dorling Kindersley India Pvt. Ltd.. p. 432. ISBN 81-317-0031-3.
- ↑ Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE (2003). "Progesterone receptors mediate male aggression toward infants". Proc. Natl. Acad. Sci. U.S.A. 100 (5): 2951–6. doi:10.1073/pnas.0130100100. PMID 12601162.
- ↑ Roof RL, Hall ED (May 2000). "Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone". J. Neurotrauma 17 (5): 367–88. doi:10.1089/neu.2000.17.367. PMID 10833057.
- ↑ Gibson CL, Gray LJ, Bath PM, Murphy SP (February 2008). "Progesterone for the treatment of experimental brain injury; a systematic review". Brain 131 (Pt 2): 318–28. doi:10.1093/brain/awm183. PMID 17715141.
- ↑ Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG (April 2007). "ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury". Ann Emerg Med 49 (4): 391–402, 402.e1–2. doi:10.1016/j.annemergmed.2006.07.932. PMID 17011666.
- ↑ Xiao G, Wei J, Yan W, Wang W, Lu Z (April 2008). "Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial". Crit Care 12 (2): R61. doi:10.1186/cc6887. PMID 18447940.
- ↑ Pan DS, Liu WG, Yang XF, Cao F (October 2007). "Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury". Biomed. Environ. Sci. 20 (5): 432–8. PMID 18188998.
Additional images
|
External links
- MeSH Progesterone
- Kimball JW (2007-05-27). "Progesterone". Kimball's Biology Pages. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/P/Progesterone.html. Retrieved 2008-06-18.
- "Progesterone Resource Center". PMS, Menopause, and Progesterone Resource Center. Oasis Advanced Wellness, Inc. http://www.pms-menopause-progesterone.org/progesterone/. Retrieved 2008-06-18.
- Infertility - possible Treatments and Causes
- Ovulation and How it happens, Triggers and How to Help it Occur
- General discussion document on Progesterone, its uses and applications
|
|||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||