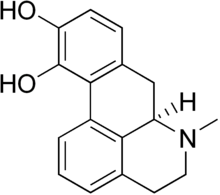
Apomorphine
Apomorphine (Apokyn, Ixense, Spontane, Uprima) is a non-selective dopamine agonist which activates both D1-like and D2-like receptors, with some preference for the latter subtypes.[1] It is historically a morphine decomposition product by boiling with concentrated acid, hence the -morphine suffix. Apomorphine does not actually contain morphine or its skeleton, or bind to opioid receptors for that matter. The apo- prefix relates to it being an aporphine derivative.
Historically, apomorphine has been tried for a variety of uses including psychiatric treatment of homosexuality in the early 20th century, and more recently in treating erectile dysfunction. Currently, apomorphine is used in the treatment of Parkinson's disease. It was also successfully used in the treatment of heroin addiction, a purpose for which it was championed by the author William S. Burroughs. It is a potent emetic (i.e. it induces vomiting) and should not be administered without an antiemetic such as domperidone. The emetic properties of apomorphine are exploited in veterinary medicine to induce therapeutic emesis in canines that have recently ingested toxic or foreign substances.
For treatment of erectile dysfunction, it is believed that dopamine receptors in the hypothalamic region of the brain are the main target, as although dopamine receptors in the penis do facilitate erection, they do so far more weakly than those in the brain.[2]
Uses
Parkinson's disease
First mooted as a treatment for Parkinson's disease as early as 1951,[3] its clinical use was first reported in 1970 by Cotzias et al.,[4] although its emetic properties and short half-life made oral use impractical. A later study found that combining the drug with the antiemetic domperidone improved results significantly.[5]
Therapeutic use in Parkinson's disease is effective because of the drug's strong dopaminergic action. When administered subcutaneously, apomorphine is the most effective dopamine agonist. Within 3–20 minutes of injection apomorphine demonstrates a magnitude of effect (ability to convert the patient with Parkinson's disease to the "on" state) that is comparable to l-dopa. A single subcutaneous injection lasts for up to 90 minutes.[6] While apomorphine can be used in combination with l-dopa, the intention is usually to reduce the l-dopa dosing, as by this stage the patient with Parkinson's disease will probably be experiencing a great deal of dopa-induced dyskinesias and "off" periods.[6] Following a successful apomorphine challenge, training of patient and caregiver, and careful dose titration, the patient can be maintained in the "on" state by the use of an apomorphine pump as an effective monotherapy.[6]
Erectile dysfunction
Apomorphine hydrochloride (trade name "Uprima", "Ixense") was a therapy used in the treatment of erectile dysfunction (male impotence). It is its mode of stimulating dopamine in the brain which is believed to enhance the sexual response. It was found to be of poor efficacy[7] in a large-scale study by Researchers at the UK's Drug Safety Research Unit and University of Portsmouth and discontinued in the UK in January 2006.[7] Around 65-70% of doctors felt it was ineffective, with 60% of over 11,000 patients (avg age 61) discontinuing in month 1 and a further 23% in month 2.[7][8] UK studies concentrated on males with generalized erectile dysfunction. Uprima effects desire and is not meant to produce an overall effect as say Viagra which works on blood flow. In those males who have problems with desire as opposed to generalized erectile dysfunction it works as expected.
Alzheimer's disease
Apomorphine has been reported [9] to be an inhibitor of Beta amyloid fibril formation, and may thus have potential as a therapeutic for Alzheimer's disease.
Pharmacology
Apomorphine possesses affinity for the following receptors:[1]
- D1 (Ki = 372 nM)
- D2S (Ki = 35 nM)
- D2L (Ki = 83 nM)
- D3 (Ki = 26 nM)
- D4 (Ki = 4.4 nM)
- D5 (Ki = 15 nM)
|
- 5-HT1A (Ki = 117 nM)
- 5-HT2A (Ki = 120 nM)
- 5-HT2B (Ki = 132 nM)
- 5-HT2C (Ki = 102 nM)
|
- α1B-adrenergic (Ki = 676 nM)
- α1D-adrenergic (Ki = 65 nM)
- α2A-adrenergic (Ki = 141 nM)
- α2B-adrenergic (Ki = 66 nM)
- α2C-adrenergic (Ki = 36 nM)
|
It has > 1,000 nM affinity for 5-HT1B, 5-HT1D, and α1A-adrenergic, and > 10,000 nM affinity for β-adrenergic, H1, and mACh.[1]
Apomorphine behaves as a partial agonist at D2S (IA = 79%), D2L (IA = 53%), D3 (IA = 82%), and D4 (IA = 45%), and as an antagonist at 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, α1-adrenergic, and α2-adrenergic.[10][11] Though its efficacies at D1 and D5 are unclear, it is known to act as an agonist at these sites.[12]
Chemistry
Properties
Apomorphine is colourless as a liquid but stains green. Therefore care must be taken to avoid splashes. Apormophine does not remain stable for more than 24 hours in a plastic container, so syringes are discarded if not used within 24 hours.
Synthesis
Apomorphine hydrochloride is synthesized by heating stoichiometric amounts of morphine and concentrated hydrochloric acid at 140ºC.
See also
References
- ↑ 1.0 1.1 1.2 Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12388666.
- ↑ Matsumoto K, Yoshida M, Andersson K, Hedlund P (2005). "Effects in vitro and in vivo by apomorphine in the rat corpus cavernosum.". Br J Pharmacol 146 (2): 259–67. doi:10.1038/sj.bjp.0706317. PMID 16025145.
- ↑ Schwab R, Amador L, Lettvin J. "Apomorphine in Parkinson's disease.". Trans Am Neurol Assoc 56: 251–3. PMID 14913646.
- ↑ Cotzias G, Papavasiliou P, Fehling C, Kaufman B, Mena I (1970). "Similarities between neurologic effects of L-dopa and of apomorphine.". N Engl J Med 282 (1): 31–3. PMID 4901383.
- ↑ Corsini G, Del Zompo M, Gessa G, Mangoni A (1979). "Therapeutic efficacy of apomorphine combined with an extracerebral inhibitor of dopamine receptors in Parkinson's disease.". Lancet 1 (8123): 954–6. doi:10.1016/S0140-6736(79)91725-2. PMID 87620.
- ↑ 6.0 6.1 6.2 Chaudhuri K, Clough C (1998). "Subcutaneous apomorphine in Parkinson's disease.". BMJ 316 (7132): 641. PMID 9522772. http://bmj.bmjjournals.com/cgi/content/full/316/7132/641#B2.
- ↑ 7.0 7.1 7.2 Pharmaceutical Business Review, "Study shows Abbott's Uprima ineffective for most UK patients"
- ↑ MedicineNet study review
- ↑ Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJE (2002). "New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease". J Biol Chem 277 (45): 42881–42890. doi:10.1074/jbc.M206593200. PMID 12167652. http://www.jbc.org/cgi/content/abstract/277/45/42881.
- ↑ Newman-Tancredi A, Cussac D, Audinot V, et al. (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12388667.
- ↑ Newman-Tancredi A, Cussac D, Quentric Y, et al. (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 815–22. doi:10.1124/jpet.102.039883. PMID 12388668. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12388668.
- ↑ Hsieh GC, Hollingsworth PR, Martino B, et al. (January 2004). "Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine". The Journal of Pharmacology and Experimental Therapeutics 308 (1): 330–8. doi:10.1124/jpet.103.057455. PMID 14569075. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=14569075.
External links
|
Antiparkinson agents (N04) |
|
| Dopaminergics |
|
|
Etilevodopa • Droxidopa • Levodopa • Melevodopa |
|
|
DA receptor agonists
|
Aplindore • Apomorphine • Bromocriptine • Cabergoline • Ciladopa • Dihydroergocryptine • Lisuride • Pardoprunox • Pergolide • Piribedil • Pramipexole • Ropinirole • Rotigotine
|
|
|
|
Ladostigil • Lazabemide • Mofegiline • Pargyline • Rasagiline • Selegiline |
|
|
COMT inhibitors
|
Entacapone • Tolcapone
|
|
|
AAAD inhibitors
|
Benserazide • Carbidopa • Methyldopa
|
|
|
| Anticholinergics |
Benzatropine • Biperiden • Bornaprine • Chlorphenoxamine • Cyrimine • Dexetimide • Dimenhydrinate • Diphenhydramine • Etanautine • Etybenzatropine • Mazaticol • Metixene • Orphenadrine • Phenglutarimide • Piroheptine • Procyclidine • Profenamine • Trihexyphenidyl • Tropatepine |
|
| Others |
Amantadine • Budipine • Memantine • Rimantadine
|
|
|
|
anat(s,m,p,,e,b,d,c,,f,,g)/phys/devp/cell
|
noco(m,d,e,h,v,s)/cong/tumr,sysi/,injr
|
proc,drug(N1A/2AB/C/3/4/7A/B//)
|
|
|
|
|
Urologicals, including antispasmodics (G04B) |
|
| Acidifiers |
|
|
Urinary antispasmodics
(primarily antimuscarinics) |
Darifenacin · Emepronium · Fesoterodine · Flavoxate · Imidafenacin · Meladrazine · Oxybutynin · Propiverine · Solifenacin · Terodiline · Tolterodine · Trospium
|
|
| For erectile dysfunction |
|
|
Alprostadil
|
|
|
PDE5 inhibitors
|
Avanafil · Sildenafil · Tadalafil · Udenafil · Vardenafil |
|
|
Alpha blockers
|
Moxisylyte · Phentolamine · Yohimbine
|
|
|
Dopamine D4 receptor agonists
|
ABT-670 · ABT-724
|
|
|
Others
|
Apomorphine · Papaverine
|
|
|
| Other urologicals |
urea analogues: Acetohydroxamic acid · Salicylhydroxamic acid
SSRI for PE: Dapoxetine
other: Collagen · Dimethyl sulfoxide · Magnesium hydroxide · Pentosan polysulfate · Phenazopyridine · Phenyl salicylate · Succinimide
|
|
|
|
|
noco/acba//tumr, sysi/, urte
|
proc/itvp, drug (G4B), blte,
|
|
|
|
|
Adrenergics |
|
|
Receptor ligands |
|
|
α1
|
Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.
|
|
|
α2
|
Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.
|
|
|
|
Agonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • N-Isopropyloctopamine • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • Xipranolol
|
|
|
|
|
Reuptake inhibitors |
|
|
NET
|
Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tramadol • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine ( Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH
|
3,4-Dihydroxystyrene
|
|
|
TH
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
DBH
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
PNMT
|
CGS-19281A • SKF-64139 • SKF-7698
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.
|
|
|
COMT
|
Entacapone • Tolcapone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Dopaminergics |
|
|
Receptor ligands |
|
|
Agonists
|
Adamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635
|
|
|
Antagonists
|
Typical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Ocaperidone • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,566 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • Yohimbine
|
|
|
|
|
Reuptake inhibitors |
|
|
|
|
DAT inhibitors
|
Piperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane ( 123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tripelennamine
|
|
|
|
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH inhibitors
|
3,4-Dihydroxystyrene
|
|
|
TH inhibitors
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD / DDC inhibitors
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
|
|
|
COMT inhibitors
|
Entacapone • Tolcapone
|
|
|
DBH inhibitors
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity Enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Serotonergics |
|
|
5-HT1 receptor ligands |
|
|
5-HT1A
|
Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • Lu AA21004 • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92016A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • Xylamidine
|
|
|
5-HT1B
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • Yohimbine
|
|
|
5-HT1D
|
Agonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • Ziprasidone
|
|
|
5-HT1E
|
Agonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/Methiothepin
|
|
|
5-HT1F
|
Agonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin
|
|
|
|
|
5-HT2 receptor ligands |
|
|
|
5-HT2A
|
Agonists: Lysergamides: ALD-52 • Ergonovine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Methysergide; Phenethylamines: 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • Yohimbine
|
|
|
5-HT2B
|
Agonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • Yohimbine
|
|
|
5-HT2C
|
Agonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Lu AA24530 • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine
|
|
|
|
|
5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands |
|
|
|
5-HT3
|
Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • Lu AA21004 • Lu AA24530 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Thujone • Xenon
|
|
|
5-HT4
|
Agonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Zacopride; Others: 5-MT • BIMU-8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • TD-5108
Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186
|
|
|
5-HT5A
|
Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.
|
|
|
5-HT6
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • N-Methyl-5-HT • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • EGIS-12233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro 04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457
|
|
|
5-HT7
|
Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507
|
|
|
|
|
Reuptake inhibitors |
|
|
SERT
|
Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lu AA21004 • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Vilazodone • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorpheniramine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Meperidine (Pethidine) • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefrine • Roxindole • SB-649,915 • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Aminoindanes: 5-IAI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Diethylcathinone • Dimethylcathinone • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • NAP • Norfenfluramine • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pFPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • Viqualine
|
|
|
|
Enzyme inhibitors |
|
|
|
|
TPH
|
AGN-2979 • Fenclonine
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Reuptake enhancers: Tianeptine
|
|
|
|