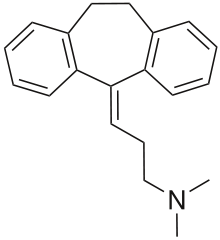
Amitriptyline
Amitriptyline (Elavil, Tryptizol, Laroxyl, Sarotex, Lentizol) is a tricyclic antidepressant (TCA).[1] It is the most widely used TCA and has at least equal efficacy against depression as the newer class of SSRIs.
History
Amitriptyline, under the brand name Elavil, was developed by Merck and approved by the FDA on April 7, 1961 for the treatment of major depression in the United States.[2] It has seen widespread usage throughout the world ever since.
Indications
Approved
Amitriptyline is approved for the treatment of major depression, as well as clinical/endogenous depression and also involutional melancholia or "depression of late life", which is no longer seen as a disease in its own right. Adult typical dosages are 25 to 150 mg daily, with half this dose initially for elderly or adolescent patients.
Children between the ages of 7 to 10 years typically have a dose of 10 to 20 mg; older children 25 to 50 mg at night. It should be gradually withdrawn at the end of the course, which overall should be of no more than three months.[3]
Amitriptyline is used in ankylosing spondylitis for pain relief and in some European countries it is officially approved as a preventive for patients with frequent/chronic migraines, usually 25 to 75 mg. It is also used as a preventive for patients with recurring biliary dyskinesia (sphincter of Oddi dysfunction), usually 10 mg daily[4].
Unapproved/off-label
Amitriptyline may be prescribed for other conditions such as insomnia, post-traumatic stress disorder (PTSD),[5] migraine, rebound headache, chronic pain, tinnitus, chronic cough, postherpetic neuralgia (persistent pain following a shingles attack), carpal tunnel syndrome (CTS), fibromyalgia, vulvodynia, interstitial cystitis, male chronic pelvic pain syndrome, irritable bowel syndrome (IBS), diabetic peripheral neuropathy, neurological pain, laryngeal sensory neuropathy, and painful paresthesias related to multiple sclerosis and at low doses as a prophylaxis (preventive) for patients with chronic migraines.[6] Typically lower dosages are required for pain modification of 10 to 50 mg daily.[3]
Amitriptyline in low doses is also sometimes prescribed to help ease the symptoms of chronic fatigue syndrome. It is thought to help combat symptoms of insomnia primarily, in addition to other selected symptoms of the affliction. Additionally, Amitriptyline is often used to relieve the pain of the Herpes Zoster virus, typically at a dose of between 10–20 mg at night.
A randomized controlled trial published in June 2005 found that amitriptyline was effective in functional dyspepsia that did not respond to a first-line treatment (famotidine or mosapride).[7]
Pharmacology
Amitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor, with strong actions on the norepinephrine transporter, and moderate effects on the serotonin transporter.[8][9] It has negligible influence on the dopamine transporter and therefore does not affect dopamine reuptake, being nearly 1,000 times weaker on it than on serotonin.[9]
Amitriptyline additionally functions as a 5-HT2A, 5-HT2C, 5-HT6, 5-HT7, α1-adrenergic, H1, and mACh receptor antagonist, and σ1 receptor agonist.[10][11][12][13] It has also been shown to be a relatively weak NMDA receptor negative allosteric modulator at the same binding site as phencyclidine.[14] Amitriptyline inhibits sodium channels, L-type calcium channels, and Kv1.1, Kv7.2, and Kv7.3 voltage-gated potassium channels, and therefore acts as a sodium, calcium, and potassium channel blocker as well.[15][16][17]
Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors.[18] It promotes the heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models.[18]
Side effects
Common side effects of using amitriptyline are mostly due to its anticholinergic activity, including: weight gain, dry mouth, changes in appetite, drowsiness, muscle stiffness, nausea, constipation, nervousness, dizziness, blurred vision, urinary retention, insomnia and changes in sexual function. Some rare side effects include tinnitus, hypotension, mania, psychosis, sleep paralysis, hypnagogia, hypnopompia, heart block, arrhythmias, lip and mouth ulcers, extrapyramidal symptoms, depression, and hepatic toxicity.
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other TCAs.
See also
References
- ↑ Barbui C, Hotopf M (February 2001). "Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials". The British Journal of Psychiatry : the Journal of Mental Science 178: 129–44. doi:10.1192/bjp.178.2.129. PMID 11157426. http://bjp.rcpsych.org/cgi/pmidlookup?view=long&pmid=11157426.
- ↑ Fangmann P, Assion HJ, Juckel G, González CA, López-Muñoz F (February 2008). "Half a century of antidepressant drugs: on the clinical introduction of monoamine oxidase inhibitors, tricyclics, and tetracyclics. Part II: tricyclics and tetracyclics". Journal of Clinical Psychopharmacology 28 (1): 1–4. doi:10.1097/jcp.0b013e3181627b60. PMID 18204333. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0271-0749&volume=28&issue=1&spage=1.
- ↑ 3.0 3.1 British National Formulary 45 (March 2003).
- ↑ S. G. Hubscher et al. (2006). Functional biliary type pain syndrome. In P. J. Pasricha, W. D. Willis & G. F. Gebhart (Eds.), ' ' italics' ' Chronic Abdominal and Visceral Pain' 'italics' '. London: Informa Healthcare, pp. 459-461.
- ↑ National Institute for Clinical Excellence: The Treatment of PTSD in Adults and Children
- ↑ Ziegler D, Hurwitz A, Hassanein R, Kodanaz H, Preskorn S, Mason J (1987). "Migraine prophylaxis. A comparison of propranolol and amitriptyline". Arch Neurol 44 (5): 486–9. PMID 3579659.
- ↑ Otaka M, Jin M, Odashima M, et al. (June 2005). "New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline". Aliment. Pharmacol. Ther. 21 (Suppl 2): 42–6. doi:10.1111/j.1365-2036.2005.02473.x. PMID 15943846. http://www.ingentaconnect.com/content/bsc/apt/2005/00000021/A00201s2/art00008.
- ↑ http://www.cnsforum.com/content/pictures/imagebank/hirespng/antidep_uptake_specific.png
- ↑ 9.0 9.1 PMID 9537821 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ PMID 9400006 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ Alan F. Schatzberg, Charles B. (2006). Essentials of clinical psychopharmacology. American Psychiatric Pub. p. 7. ISBN 1585622435, 9781585622436.
- ↑ PMID 11561066 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ PMID 17689532 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ PMID 2568580 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ PMID 9435180 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ PMID 18048694 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- ↑ Punke MA, Friederich P (May 2007). "Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels". Anesthesia and Analgesia 104 (5): 1256–64, tables of contents. doi:10.1213/01.ane.0000260310.63117.a2. PMID 17456683. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=17456683.
- ↑ 18.0 18.1 PMID 19549602 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
Further reading
|
Antidepressants (N06A) |
|
|
Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) |
|
|
|
|
|
|
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
|
|
|
|
Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs)
|
Brasofensine · BTS-74,398 · Cocaine · Diclofensine · DOV-21,947 · DOV-102,677 · DOV-216,303 · EXP-561 · Fezolamine · JNJ-7,925,476 · NS-2359 · PRC200-SS · Pridefrine · SEP-225,289 · SEP-227,162 · Tesofensine |
|
|
Norepinephrine reuptake inhibitors (NRIs)
|
Amedalin · Atomoxetine/Tomoxetine · Binedaline · Ciclazindol · Daledalin · Esreboxetine · Lortalamine · Mazindol · Nisoxetine · Reboxetine · Talopram · Talsupram · Tandamine · Viloxazine |
|
|
Dopamine reuptake inhibitors (DRIs)
|
Medifoxamine · Vanoxerine
|
|
|
Norepinephrine-dopamine reuptake inhibitors (NDRIs)
|
|
|
|
Norepinephrine-dopamine releasing agents (NDRAs)
|
|
|
|
Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)
|
4-Methyl-αMT · αET/Etryptamine · αMT/Metryptamine
|
|
|
Selective serotonin reuptake enhancers (SSREs)
|
Tianeptine
|
|
|
Others
|
|
|
|
|
|
Receptor antagonists and/or reuptake inhibitors |
|
|
Serotonin antagonists and reuptake inhibitors (SARIs)
|
|
|
|
Noradrenergic and specific serotonergic antidepressants (NaSSAs)
|
Aptazapine · Esmirtazapine · Mianserin · Mirtazapine · Setiptiline/Teciptiline |
|
|
Norepinephrine-dopamine disinhibitors (NDDIs)
|
Agomelatine
|
|
|
Serotonin modulators and stimulators (SMSs)
|
Lu AA21004
|
|
|
|
|
Tricyclic and tetracyclic antidepressants (TCAs/TeCAs) |
|
Tricyclics: Amezepine · Amineptine · Amitriptyline · Amitriptylinoxide · Azepindole · Butriptyline · Cianopramine · Clomipramine · Cotriptyline · Cyanodothiepin · Demexiptiline · Depramine/Balipramine · Desipramine · Dibenzepine · Dimetacrine · Dosulepin/Dothiepin · Doxepin · Enprazepine · Fluotracen · Hepzidine · Homopipramol · Imipramine · Imipraminoxide · Intriptyline · Iprindole · Ketipramine · Litracen · Lofepramine · Losindole · Mariptiline · Melitracen · Metapramine · Mezepine · Naranol · Nitroxazepine · Nortriptyline · Noxiptiline · Octriptyline · Opipramol · Pipofezine · Propizepine · Protriptyline · Quinupramine · Tampramine · Tianeptine · Tienopramine · Trimipramine; Tetracyclics: 7-OH-Amoxapine · Amoxapine · Aptazapine · Azipramine · Ciclazindol · Ciclopramine · Esmirtazapine · Loxapine · Maprotiline · Mazindol · Mianserin · Mirtazapine · Oxaprotiline · Setiptiline/Teciptiline
|
|
|
|
Monoamine oxidase inhibitors (MAOIs) |
|
Nonselective: Irreversible: Benmoxin · Echinopsidine · Iproclozide · Iproniazid · Isocarboxazid · Mebanazine · Metfendrazine · Nialamide · Octamoxin · Phenelzine · Pheniprazine · Phenoxypropazine · Pivalylbenzhydrazine · Safrazine · Tranylcypromine; Reversible: Caroxazone · Paraxazone; MAOA-Selective: Irreversible: Clorgyline; Reversible: Amiflamine · Bazinaprine · Befloxatone · Befol · Brofaromine · Cimoxatone · Esuperone · Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) · Methylene Blue · Metralindole · Minaprine · Moclobemide · Pirlindole · Sercloremine · Tetrindole · Toloxatone · Tyrima; MAOB-Selective: Irreversible: Ladostigil · Mofegiline · Pargyline · Rasagiline · Selegiline; Reversible: Lazabemide · Milacemide
|
|
|
|
Azapirones and other 5-HT1A receptor agonists |
|
Alnespirone · Aripiprazole · Befiradol · Buspirone · Eptapirone · Flesinoxan · Flibanserin · Gepirone · Ipsapirone · Oxaflozane · Tandospirone · Vilazodone · Zalospirone |
|
|
|
|
|
|
Research compounds and miscellaneous agents |
|
|
5-HT4R agonists
|
RS-67,333 · SL65.0155
|
|
|
5-HT7R antagonists
|
Amisulpride
|
|
|
|
|
|
|
β3-Adrenoceptor agonists
|
Amibegron · Solabegron
|
|
|
|
|
|
|
|
|
|
|
COMT inhibitors
|
Entacapone · Tolcapone
|
|
|
CRF1R antagonists
|
Antalarmin · CP-154,526 · Pexacerfont · Pivagabine
|
|
|
D2/D3AR antagonists
|
Amisulpride · Sulpiride
|
|
|
D2/D3/D4R agonists
|
Piribedil · Pramipexole · Ropinirole · Rotigotine · Roxindole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Agomelatine · Melatonin · Ramelteon · Tasimelteon |
|
|
NK1R antagonists
|
Aprepitant · Casopitant · Fosaprepitant · L-733,060 · Maropitant · Vestipitant
|
|
|
|
|
|
|
PDE4 inhibitors
|
Mesembrine (Kanna) · Rolipram
|
|
|
|
|
|
|
|
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Anxiolytics (N05B) |
|
| GABAA PAMs |
|
|
Adinazolam • Alprazolam • Bretazenil • Bromazepam • Camazepam • Chlordiazepoxide • Clobazam • Clonazepam • Clorazepate • Clotiazepam • Cloxazolam • Diazepam • Ethyl Loflazepate • Etizolam • Fludiazepam • Halazepam • Imidazenil • Ketazolam • Lorazepam • Medazepam • Nordazepam • Oxazepam • Pinazepam • Prazepam |
|
|
Carbamates
|
Emylcamate • Mebutamate • Meprobamate (Carisoprodol, Tybamate) • Phenprobamate • Procymate
|
|
|
Nonbenzodiazepines
|
Abecarnil • Adipiplon • Alpidem • CGS-9896 • CGS-20625 • Divaplon • ELB-139 • Fasiplon • GBLD-345 • Gedocarnil • L-838,417 • NS-2664 • NS-2710 • Ocinaplon • Pagoclone • Panadiplon • Pipequaline • RWJ-51204 • SB-205,384 • SL-651,498 • Taniplon • TP-003 • TP-13 • TPA-023 • Y-23684 • ZK-93423
|
|
|
Pyrazolopyridines
|
Cartazolate • Etazolate • ICI-190,622 • Tracazolate
|
|
|
Others
|
|
|
|
| α2δ VDCC Blockers |
|
|
| 5-HT1A Agonists |
Azapirones: Buspirone • Gepirone • Tandospirone; Others: Flesinoxan • Oxaflozane
|
|
| H1 Antagonists |
Diphenylmethanes: Captodiame • Hydroxyzine; Others: Brompheniramine • Chlorpheniramine • Pheniramine
|
|
| CRH1 Antagonists |
Antalarmin • CP-154,526 • Pexacerfont • Pivagabine
|
|
| NK2 Antagonists |
GR-159,897 • Saredutant
|
|
| MCH1 antagonists |
ATC-0175 • SNAP-94847
|
|
| mGluR2/3 Agonists |
Eglumegad
|
|
| mGluR5 NAMs |
Fenobam
|
|
| TSPO agonists |
DAA-1097 • DAA-1106 • Emapunil • FGIN-127 • FGIN-143
|
|
| σ1 agonists |
Afobazole • Opipramol
|
|
| Others |
Benzoctamine • Carbetocin • Demoxytocin • Mephenoxalone • Mepiprazole • Oxanamide • Oxytocin • Promoxolane • Tofisopam • Trimetozine • WAY-267,464 |
|
#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Hypnotics/Sedatives (N05C) |
|
| GABAA receptor |
|
|
|
Ultrashort-acting
|
|
|
|
Short/intermediate-
acting
|
Allobarbital • Amobarbital • Butabarbital • Butobarbital • Pentobarbital • Secobarbital • Talbutal
|
|
|
Long-acting
|
|
|
|
Ungrouped
|
Cyclobarbital • Ethallobarbital • Heptabarbital • Hexobarbital • Proxibarbal • Reposal • Vinylbital • Vinbarbital
|
|
|
|
|
|
Short-acting
|
Brotizolam • Cinolazepam • Doxefazepam • Loprazolam • Midazolam • Triazolam |
|
|
Intermediate-acting
|
|
|
|
Long-acting
|
Flurazepam • Flutoprazepam • Nitrazepam • Quazepam
|
|
|
|
Dialkylphenols
|
|
|
|
Nonbenzo-
diazepines
|
CL-218,872 • Eszopiclone • Indiplon • Lirequinil • Necopidem • Pazinaclone • ROD-188 • Saripidem • Suproclone • Suriclone • SX-3228 • U-89843A • U-90042 • Zaleplon • Zolpidem • Zopiclone |
|
|
Piperidinediones
|
Glutethimide • Methyprylon • Pyrithyldione • Piperidione
|
|
|
Quinazolinones
|
Afloqualone • Cloroqualone • Diproqualone • Etaqualone • Mebroqualone • Mecloqualone • Methaqualone • Methylmethaqualone • Nitromethaqualone |
|
|
Neuroactive
steroids
|
Acebrochol • Allopregnanolone • Alphadolone • Alphaxolone • Eltanolone • Ganaxolone • Hydroxydione • Minaxolone • Org 20599 • Org 21465 • Tetrahydrodeoxycorticosterone
|
|
|
Alpha-2 adrenergic
receptor |
|
Alpha-adrenergic agonists
|
4-NEMD • Clonidine • Dexmedetomidine • Lofexidine • Medetomidine • Romifidine • Tizanidine • Xylazine |
|
|
| Melatonin receptor |
|
|
Agomelatine • Melatonin • Ramelteon • Tasimelteon |
|
|
Histamine receptor &
Acetylcholine receptor |
|
|
Amitriptyline • Dimenhydrinate • Doxylamine • Hydroxyzine • Diphenhydramine • Bromodiphenhydramine • Carbinoxamine • Doxepin • Esmirtazapine • Orphenadrine • Mianserin • Mirtazapine • Phenyltoloxamine • Propiomazine • Pyrilamine • Scopolamine |
|
|
5-HT2A &
α1-adrenergic |
|
Selective 5-HT2A & α1-adrenergic antagonists
|
Etoperidone • Nefazodone • Niaprazine • Trazodone |
|
|
GABAB receptor /
GHB receptor |
|
|
| Orexin receptors |
|
Orexin antagonists
|
Almorexant • SB-334,867 • SB-408,124 • SB-649,868 • TCS-OX2-29
|
|
|
Other receptors/
ungrouped |
|
|
Acetylglycinamide chloral hydrate • Chloral hydrate • Chloralodol • Dichloralphenazone • Paraldehyde • Petrichloral
|
|
|
|
Centalun • Ethchlorvynol • Ethinamate • Hexapropymate • Methylpentynol
|
|
|
Carbamates
|
Meprobamate • Carisoprodol • Tybamate • Methocarbamol • Procymate
|
|
|
Other
|
2-Methyl-2-butanol • Acecarbromal • Acetophenone • Apronal • Bromides • Bromisoval • Carbromal • Chloralose • Clomethiazole • Embutramide • Etomidate • Evoxine • Fenadiazole • Gaboxadol • Loreclezole • Mephenoxalone • Sulfonmethane • Trichloroethanol • Triclofos • Valerian • Valnoctamide |
|
#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
|
Adrenergics |
|
|
Receptor ligands |
|
|
α1
|
Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.
|
|
|
α2
|
Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.
|
|
|
|
Agonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • N-Isopropyloctopamine • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • Xipranolol
|
|
|
|
|
Reuptake inhibitors |
|
|
NET
|
Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tramadol • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine ( Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH
|
3,4-Dihydroxystyrene
|
|
|
TH
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
DBH
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
PNMT
|
CGS-19281A • SKF-64139 • SKF-7698
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.
|
|
|
COMT
|
Entacapone • Tolcapone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Cholinergics |
|
|
Receptor ligands |
|
|
mAChR
|
Agonists: 77-LH-28-1 · AC-42 · AC-260,584 · Aceclidine · Acetylcholine · AF30 · AF150(S) · AF267B · AFDX-384 · Alvameline · AQRA-741 · Arecoline · Bethanechol · Butyrylcholine · Carbachol · CDD-0034 · CDD-0078 · CDD-0097 · CDD-0098 · CDD-0102 · Cevimeline · cis-Dioxolane · Ethoxysebacylcholine · LY-593,039 · L-689,660 · LY-2,033,298 · McNA343 · Methacholine · Milameline · Muscarine · NGX-267 · Ocvimeline · Oxotremorine · PD-151,832 · Pilocarpine · RS86 · Sabcomeline · SDZ 210-086 · Sebacylcholine · Suberylcholine · Talsaclidine · Tazomeline · Thiopilocarpine · Vedaclidine · VU-0029767 · VU-0090157 · VU-0152099 · VU-0152100 · VU-0238429 · WAY-132,983 · Xanomeline · YM-796
Antagonists: 3-Quinuclidinyl Benzilate · 4-DAMP · Aclidinium Bromide · Anisodamine · Anisodine · Atropine · Atropine Methonitrate · Benactyzine · Benzatropine (Benztropine) · Benzydamine · BIBN 99 · Biperiden · Bornaprine · CAR-226,086 · CAR-301,060 · CAR-302,196 · CAR-302,282 · CAR-302,368 · CAR-302,537 · CAR-302,668 · CS-27349 · Cyclobenzaprine · Cyclopentolate · Darifenacin · DAU-5884 · Dimethindene · Dexetimide · DIBD · Dicyclomine (Dicycloverine) · Ditran · EA-3167 · EA-3443 · EA-3580 · EA-3834 · Elemicin · Etanautine · Etybenzatropine (Ethylbenztropine) · Flavoxate · Himbacine · HL-031,120 · Ipratropium · J-104,129 · Hyoscyamine · Mamba Toxin 3 · Mamba Toxin 7 · Mazaticol · Mebeverine · Methoctramine · Metixene · Myristicin · N-Ethyl-3-Piperidyl Benzilate · N-Methyl-3-Piperidyl Benzilate · Orphenadrine · Otenzepad · Oxybutynin · PBID · PD-102,807 · Phenglutarimide · Phenyltoloxamine · Pirenzepine · Piroheptine · Procyclidine · Profenamine · RU-47,213 · SCH-57,790 · SCH-72,788 · SCH-217,443 · Scopolamine (Hyoscine) · Solifenacin · Telenzepine · Tiotropium · Tolterodine · Trihexyphenidyl · Tripitamine · Tropatepine · Tropicamide · WIN-2299 · Xanomeline · Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorpheniramine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) · Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) · Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) · Typical Antipsychotics ( Chlorpromazine, thioridazine, etc) · Atypical Antipsychotics ( Clozapine, olanzapine, quetiapine, etc)
|
|
|
nAChR
|
Agonists: 5-HIAA · A-84,543 · A-366,833 · A-582,941 · A-867,744 · ABT-202 · ABT-418 · ABT-560 · ABT-894 · Acetylcholine · Altinicline · Anabasine · AR-R17779 · Butyrylcholine · Carbachol · Cotinine · Cytisine · Decamethonium · Desformylflustrabromine · Dianicline · Dimethylphenylpiperazinium · Epibatidine · Epiboxidine · Ethanol · Ethoxysebacylcholine · EVP-4473 · EVP-6124 · Galantamine · GTS-21 · Ispronicline · Lobeline · MEM-63,908 (RG-3487) · Nicotine · NS-1738 · PHA-543,613 · PHA-709,829 · PNU-120,596 · PNU-282,987 · Pozanicline · Rivanicline · Sazetidine A · Sebacylcholine · SIB-1508Y · SIB-1553A · SSR-180,711 · Suberylcholine · TC-1698 · TC-1734 · TC-1827 · TC-2216 · TC-5214 · TC-5619 · TC-6683 · Tebanicline · Tropisetron · UB-165 · Varenicline · WAY-317,538 · XY-4083
Antagonists: 18-Methoxycoronaridine · α-Bungarotoxin · α-Conotoxin · Alcuronium · Amantadine · Anatruxonium · Atracurium · Bupropion (Amfebutamone) · Chandonium · Chlorisondamine · Cisatracurium · Coclaurine · Coronaridine · Dacuronium · Decamethonium · Dextromethorphan · Dextropropoxyphene · Dextrorphan · Diadonium · DHβE · Dimethyltubocurarine (Metocurine) · Dipyrandium · Dizocilpine (MK-801) · Doxacurium · Duador · Esketamine · Fazadinium · Gallamine · Hexafluronium · Hexamethonium (Benzohexonium) · Ibogaine · Isoflurane · Ketamine · Kynurenic acid · Laudexium (Laudolissin) · Levacetylmethadol · Malouetine · Mecamylamine · Memantine · Methadone · Methorphan (Racemethorphan) · Methyllycaconitine · Metocurine · Mivacurium · Morphanol (Racemorphanol) · Neramexane · Nitrous Oxide · Pancuronium · Pempidine · Pentamine · Pentolinium · Phencyclidine · Pipecuronium · Radafaxine · Rapacuronium · Rocuronium · Surugatoxin · Suxamethonium (Succinylcholine) · Thiocolchicoside · Toxiferine · Trimethaphan · Tropeinium · Tubocurarine · Vecuronium · Xenon
|
|
|
|
|
Reuptake inhibitors |
|
|
|
|
CHT Inhibitors
|
Hemicholinium-3 (Hemicholine; HC3) · Triethylcholine
|
|
|
|
Vesicular
|
|
VAChT Inhibitors
|
Vesamicol
|
|
|
|
|
|
Enzyme inhibitors |
|
|
|
|
ChAT inhibitors
|
1-(-Benzoylethyl)pyridinium · 2-(α-Naphthoyl)ethyltrimethylammonium · 3-Chloro-4-stillbazole · 4-(1-Naphthylvinyl)pyridine · Acetylseco hemicholinium-3 · Acryloylcholine · AF64A · B115 · BETA · CM-54,903 · N,N-Dimethylaminoethylacrylate · N,N-Dimethylaminoethylchloroacetate
|
|
|
|
|
|
AChE inhibitors
|
Reversible: Carbamates: Aldicarb · Bendiocarb · Bufencarb · Carbaryl · Carbendazim · Carbetamide · Carbofuran · Chlorbufam · Chloropropham · Ethienocarb · Ethiofencarb · Fenobucarb · Fenoxycarb · Formetanate · Furadan · Ladostigil · Methiocarb · Methomyl · Miotine · Oxamyl · Phenmedipham · Pinmicarb · Pirimicarb · Propamocarb · Propham · Propoxur; Stigmines: Ganstigmine · Neostigmine · Phenserine · Physostigmine · Pyridostigmine · Rivastigmine; Others: Acotiamide · Ambenonium · Donepezil · Edrophonium · Galantamine · Huperzine A · Minaprine · Tacrine · Zanapezil
Irreversible: Organophosphates: Acephate · Azinphos-methyl · Bensulide · Cadusafos · Chlorethoxyfos · Chlorfenvinphos · Chlorpyrifos · Chlorpyrifos-Methyl · Coumaphos · Cyclosarin (GF) · Demeton · Demeton-S-Methyl · Diazinon · Dichlorvos · Dicrotophos · Diisopropyl fluorophosphate (Guthion) · Diisopropylphosphate · Dimethoate · Dioxathion · Disulfoton · EA-3148 · Echothiophate · Ethion · Ethoprop · Fenamiphos · Fenitrothion · Fenthion · Fosthiazate · GV · Isofluorophate · Isoxathion · Malaoxon · Malathion · Methamidophos · Methidathion · Metrifonate · Mevinphos · Monocrotophos · Naled · Novichok agent · Omethoate · Oxydemeton-Methyl · Paraoxon · Parathion · Parathion-Methyl · Phorate · Phosalone · Phosmet · Phostebupirim · Phoxim · Pirimiphos-Methyl · Sarin (GB) · Soman (GD) · Tabun (GA) · Temefos · Terbufos · Tetrachlorvinphos · Tribufos · Trichlorfon · VE · VG · VM · VR · VX; Others: Demecarium · Onchidal ( Onchidella binneyi)
|
|
|
BChE inhibitors
|
* Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.
|
|
|
|
|
|
Others |
|
|
Precursors
|
Choline (Lecithin) · Citicoline · Cyprodenate · Dimethylethanolamine (DMAE, deanol) · Glycerophosphocholine · Meclofenoxate (Centrophenoxine) · Phosphatidylcholine · Phosphatidylethanolamine · Phosphorylcholine · Pirisudanol
|
|
|
|
|
|
|
Others
|
Acetylcholine releasing agents: α-Latrotoxin · β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime · Obidoxime · Pralidoxime
|
|
|
|
|
Glutamatergics |
|
| Ionotropic |
|
AMPA
|
Agonists: 5-Fluorowillardiine • AMPA • Domoic acid • Quisqualic acid; Positive allosteric modulators: Aniracetam • Cyclothiazide • CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • Diazoxide • HCTZ • IDRA-21 • LY-392,098 • LY-404,187 • LY-451,395 • LY-451,646 • LY-503,430 • Oxiracetam • PEPA • Piracetam • Pramiracetam • S-18986 • Sunifiram • Unifiram
Antagonists: ATPO • Barbiturates • Caroverine • CNQX • DNQX • GYKI-52466 • NBQX • Perampanel • Talampanel • Tezampanel • Topiramate; Negative allosteric modulators: GYKI-53,655
|
|
|
|
Agonists: Glutamate/acite site competitive agonists: Aspartate • Glutamate • Homoquinolinic acid • Ibotenic acid • NMDA • Quinolinic acid • Tetrazolylglycine; Glycine site agonists: ACBD • ACPC • ACPD • Alanine • CCG • Cycloserine • DHPG • Fluoroalanine • Glycine • HA-966 • L-687,414 • Milacemide • Sarcosine • Serine • Tetrazolylglycine; Polyamine site agonists: Acamprosate • Spermidine • Spermine
Antagonists: Competitive antagonists: AP5 (APV) • AP7 • CGP-37849 • CGP-39551 • CGP-39653 • CGP-40116 • CGS-19755 • CPP • LY-233,053 • LY-235,959 • LY-274,614 • MDL-100,453 • Midafotel (d-CPPene) • NPC-12,626 • NPC-17,742 • PBPD • PEAQX • Perzinfotel • PPDA • SDZ-220581 • Selfotel; Noncompetitive antagonists: ARR-15,896 • Caroverine • Dexanabinol • FPL-12495 • FR-115,427 • Hodgkinsine • Magnesium • MDL-27,266 • NPS-1506 • Psychotridine • Zinc; Uncompetitive pore blockers: 2-MDP • 3-MeO-PCP • 8A-PDHQ • Amantadine • Aptiganel • ARL-12,495 • ARL-15,896-AR • ARL-16,247 • Budipine • Delucemine • Dexoxadrol • Dextrallorphan • Dieticyclidine • Dizocilpine • Endopsychosin • Esketamine • Etoxadrol • Eticyclidine • Gacyclidine • Ibogaine • Indantadol • Ketamine • Ketobemidone • Loperamide • Memantine • Meperidine (Pethidine) • Methadone • Methorphan ( Dextromethorphan, Levomethorphan) • Methoxetamine • Milnacipran • Morphanol (Dextrorphan, Levorphanol) • NEFA • Neramexane • Nitrous oxide • Noribogaine • Orphenadrine • PCPr • Phencyclamine • Phencyclidine • Propoxyphene • Remacemide • Rhynchophylline • Riluzole • Rimantadine • Rolicyclidine • Sabeluzole • Tenocyclidine • Tiletamine • Tramadol • Xenon; Glycine site antagonists: ACEA-1021 • ACEA-1328 • ACPC • Carisoprodol • CGP-39653 • CKA • DCKA • Felbamate • Gavestinel • GV-196,771 • Kynurenic acid • L-689,560 • L-701,324 • Lacosamide • Licostinel • LU-73,068 • MDL-105,519 • Meprobamate • MRZ 2/576 • PNQX • ZD-9379; NR2B subunit antagonists: Besonprodil • CO-101,244 (PD-174,494) • CP-101,606 • Eliprodil • Haloperidol • Ifenprodil • Isoxsuprine • Nylidrin • Ro8-4304 • Ro25-6981 • Traxoprodil; Polyamine site antagonists: Arcaine • Co 101676 • Diaminopropane • Diethylenetriamine • Huperzine A • Putrescine • Ro 25-6981; Unclassified/unsorted antagonists: Chloroform • Diethyl ether • Enflurane • Ethanol (Alcohol) • Halothane • Isoflurane • Methoxyflurane • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • Xylene
|
|
|
Kainate
|
Agonists: 5-Iodowillardiine • ATPA • Domoic acid • Kainic acid • LY-339,434 • SYM-2081
Antagonists: CNQX • DNQX • LY-382,884 • NBQX • NS102 • Tezampanel • Topiramate • UBP-302; Negative allosteric modulators: NS-3763
|
|
|
| Metabotropic |
|
Group I
|
Agonists: Unselective: ACPD • DHPG • Quisqualic acid; mGlu1-selective: Ro01-6128 • Ro67-4853 • Ro67-7476 • VU-71; mGlu5-selective: ADX-47273 • CDPPB • CHPG • DFB • VU-1545
Antagonists: Unselective: MCPG • NPS-2390; mGlu1-selective: BAY 36-7620 • CPCCOEt • LY-367,385 • LY-456,236; mGlu5-selective: DMeOB • Fenobam • LY-344,545 • MPEP • MTEP • SIB-1757 • SIB-1893
|
|
|
Group II
|
Agonists: Unselective: CBiPES • DCG-IV • Eglumegad • LY-379,268 • LY-404,039 • LY-487,379 • MGS-0028; mGlu2-selective: BINA • LY-566,332
Antagonists: Unselective: APICA • EGLU • HYDIA • LY-307,452 • LY-341,495 • MCPG: mGlu2-selective: PCCG-4; mGlu3-selective: CECXG
|
|
|
Group III
|
Agonists: Unselective: L-AP4; mGlu4-selective: PHCCC • VU-001,171 • VU-0155,041; mGlu7-selective: AMN082; mGlu8-selective: DCPG
Antagonists: Unselective: CPPG • MAP4 • MSOP • MPPG • MTPG • UBP-1112; mGlu7-selective: MMPIP
|
|
|
Transporter
inhibitors |
|
EAATs
|
DHKA • PDC • WAY-213,613
|
|
|
vGluTs
|
7-CKA • Evans blue
|
|
|
|
Histaminergics |
|
Receptor
ligands |
|
H1
|
Agonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorpheniramine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; 3rd generation: Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic Antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic Antidepressants (Mianserin, Mirtazapine, etc) • Serotonin Antagonists and Reuptake Inhibitors ( Trazodone, Nefazodone) • Typical Antipsychotics ( Chlorpromazine, Thioridazine, etc) • Atypical Antipsychotics ( Clozapine, Olanzapine, Quetiapine, etc)
|
|
|
H2
|
Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Bisfentidine • Burimamide • Cimetidine • Dalcotidine • Donetidine • Ebrotidine • Etintidine • Famotidine • Lafutidine • Lamtidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Mifentidine • Niperotidine • Nizatidine • Osutidine • Oxmetidine • Pibutidine • Quisultidine/Quisultazine • Ramixotidine • Ranitidine • Roxatidine • Sufotidine • Tiotidine • Tuvatidine • Venritidine • Zaltidine
|
|
|
H3
|
Agonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • SCH-79,687 • Thioperamide • Tiprolisant • VUF-5,681
|
|
|
H4
|
Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002
|
|
|
Reuptake
inhibitors |
|
|
Enzyme
inhibitors |
|
|
|
HDC inhibitors
|
α-FMH • Brocresine • Catechin • Cyanidanol-3 • McN-A-1293 • ME • Meciadanol • Naringenin • Thiazol-4-yimethoxyamine • Tritoqualine • Zy-15,029
|
|
|
|
|
|
HNMT inhibitors
|
Amodiaquine • BW-301U • Diphenhydramine • Harmaline • Metoprine • Quinacrine • SKF-91,488 • Tacrine |
|
|
DAO inhibitors
|
1,4-Diamino-2-butyne • Aminoguanidine
|
|
|
|
| Others |
|
|
|
Serotonergics |
|
|
5-HT1 receptor ligands |
|
|
5-HT1A
|
Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • Lu AA21004 • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92016A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • Xylamidine
|
|
|
5-HT1B
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • Yohimbine
|
|
|
5-HT1D
|
Agonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • Ziprasidone
|
|
|
5-HT1E
|
Agonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/Methiothepin
|
|
|
5-HT1F
|
Agonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin
|
|
|
|
|
5-HT2 receptor ligands |
|
|
|
5-HT2A
|
Agonists: Lysergamides: ALD-52 • Ergonovine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Methysergide; Phenethylamines: 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • Yohimbine
|
|
|
5-HT2B
|
Agonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • Yohimbine
|
|
|
5-HT2C
|
Agonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Lu AA24530 • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine
|
|
|
|
|
5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands |
|
|
|
5-HT3
|
Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • Lu AA21004 • Lu AA24530 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Thujone • Xenon
|
|
|
5-HT4
|
Agonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Zacopride; Others: 5-MT • BIMU-8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • TD-5108
Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186
|
|
|
5-HT5A
|
Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.
|
|
|
5-HT6
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • N-Methyl-5-HT • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • EGIS-12233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro 04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457
|
|
|
5-HT7
|
Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507
|
|
|
|
|
Reuptake inhibitors |
|
|
SERT
|
Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lu AA21004 • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Vilazodone • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorpheniramine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Meperidine (Pethidine) • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefrine • Roxindole • SB-649,915 • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Aminoindanes: 5-IAI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Diethylcathinone • Dimethylcathinone • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • NAP • Norfenfluramine • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pFPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • Viqualine
|
|
|
|
Enzyme inhibitors |
|
|
|
|
TPH
|
AGN-2979 • Fenclonine
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Reuptake enhancers: Tianeptine
|
|
|
|
|
Tricyclics |
|
| Classes |
Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • Thioxanthene |
|
| Antidepressants |
7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepine • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • Trimipramine |
|
| Antihistamines |
Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • Thiazinamium
|
|
| Antipsychotics |
Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • Zuclopenthixol |
|
| Others |
Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • Profenamine |
|