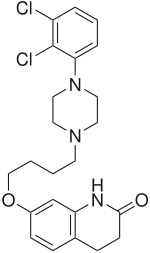
Aripiprazole
Aripiprazole (pronounced AR-i-PIP-ra-zole; brand names: Abilify, Abilify Discmelt) is an atypical antipsychotic and antidepressant used in the treatment of schizophrenia, bipolar disorder, and clinical depression. It was approved by the US Food and Drug Administration (FDA) for schizophrenia on November 15, 2002, for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004, and as an adjunct for major depressive disorder on November 20, 2007.[1] Aripiprazole was developed by Otsuka in Japan, and in the United States, Otsuka America markets it jointly with Bristol-Myers Squibb.
Indications and usage
Schizophrenia
Aripiprazole has been approved by the FDA for the treatment of schizophrenia.[2]
Bipolar disorder
Aripiprazole has been approved by the FDA for the treatment of acute manic and mixed episodes, in both pediatric patients aged 10–17 and in adults.[3] Several double-blind, placebo-controlled trials support this use.[4][5][6][7] In addition, it is often used as maintenance therapy, either on its own or in conjunction with a mood stabilizer such as lithium or valproate. This use is also supported by a handful of studies.[8][9] Aripiprazole is at least as effective as haloperidol at reducing manic symptoms,[10] and is much better tolerated by patients.[11]
Aripiprazole's use as a monotherapy in bipolar depression is more controversial. While a few pilot studies have found some effectiveness[12][13] (with one finding a reduction in anhedonia symptoms[14]), two large, double-blind, placebo-controlled studies found no difference between aripiprazole and placebo.[15] One study reported depression as a side effect of the drug.[16]
Major depression (Unipolar depression)
In 2007, aripiprazole was approved by the FDA for the treatment of unipolar depression when used adjunctively with an antidepressant medication.[17] It has not been FDA-approved for use as monotherapy in unipolar depression.
Autism
In 2009, the United States FDA approved Abilify to treat irritability in persons with autism.[18] It was approved on the basis of two studies that showed it reduced aggression towards others, self-injury, quickly changing moods, irritability, and temper tantrums in autistic males and females 6–17 years of age.
Cocaine dependency
Perhaps owing to its mechanism of action relating to dopamine receptors, there is some evidence to suggest that aripiprazole blocks cocaine-seeking behaviour in animal models without significantly affecting other rewarding behaviours (such as food self-administration). [19]
Pharmacology
Aripiprazole's mechanism of action is different from those of the other FDA-approved atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, ziprasidone, and risperidone). Rather than antagonizing the D2 receptor, aripiprazole acts as a D2 partial agonist.[20][21] Aripiprazole is also a partial agonist at the 5-HT1A receptor, and like the other atypical antipsychotics displays an antagonist profile at the 5-HT2A receptor.[22][23] It also antagonizes the 5-HT7 receptor and acts as a partial agonist at the 5-HT2C receptor, both with high affinity. The latter action may underlie the minimal weight gain seen in the course of therapy.[24] Aripiprazole has moderate affinity for histamine and α-adrenergic receptors and for the serotonin transporter, and no appreciable affinity for cholinergic muscarinic receptors.[25]
D2 and D3 receptor occupancy levels are high, with average levels ranging between ~71% at 2 mg/day to ~96% at 40 mg/day.[26][27] Most atypical antipsychotics bind preferentially to extrastriatal receptors, but aripiprazole appears to be less preferential in this regard, as binding rates are high throughout the brain.[28]
Recently, it has been demonstrated that in 5-HT7 receptor knockout mice, aripiprazole does not reduce immobility time in the forced swim test (FST), and actually increases it.[29][30] This implicates 5-HT7 antagonism as playing a major role in aripiprazole's antidepressant effects, similarly to amisulpride.[29][30][31]
Aripiprazole produces 2,3-dichlorophenylpiperazine (DCPP) as a metabolite similarly to how trazodone and nefazodone reduce to 3-chlorophenylpiperazine (mCPP) and niaprazine converts to 4-fluorophenylpiperazine (pFPP).[32] It is unknown whether DCPP contributes to aripiprazole's pharmacology in any way, but the possibility cannot be excluded.
Pharmacokinetics
Aripiprazole displays linear kinetics and has an elimination half-life of approximately 75 hours. Steady-state plasma concentrations are achieved in about 14 days. Cmax (maximum plasma concentration) is achieved 3–5 hours after oral dosing. Bioavailability of the oral tablets is about 90% and the drug undergoes extensive hepatic metabolization (dehydrogenation, hydroxylation, and N-dealkylation), principally by the enzymes CYP2D6 and CYP3A4. Its only known active metabolite is dehydro-aripiprazole, which typically accumulates to approximately 40% of the aripiprazole concentration. The parenteral drug is excreted only in traces, and its metabolites, active or not, are excreted via feces and urine.[25] When dosed daily, brain concentrations of aripiprazole will increase for a period of 10-14 days, before reaching stable constant levels. This phenomenon is due to the long half life of aripiprazole, and is responsible for many of the adverse side effects that appear after multiple days of dosing (whereas the first dose normally does not cause these side effects). Aripiprazole possesses very high binding affinity for D2 receptors throughout the brain. Although aripiprazole is described as a partial agonist, its intrinisic affinity is lower than most other partial agonists, thus functioning as an antagonist in most physiological instances. This is due to the normal presence of dopamine throughout the brain, which possesses a much higher intrinsic activity for dopamine receptors. Single low doses of ariprazole will only occupy small amounts of dopamine D2 receptors, which does not cause extrapyramidial symptoms, akathisia, or parkinsonism. However, even if the dose is low, consecutive daily administration will result in accumulation of brain concentrations, thereby causing undesirable side effects in many patients, such as Akathisia, anxiety, restless leg syndrome, and other side effects typical of traditional and atypical antipsychotic medications. An even higher risk for unwanted side effects, is present in situations of high-dose daily dosing (10mg and above/per day). Such dosing results in brain concentrations occupying up to 80% of dopamine D2 receptors in most areas of the brain. Common medical knowledge assumes that due to the partial agonist properties of aripiprazole, typical D2-blocking side effects will not be significant. This assumption is incorrect: a partial agonist will always produce agonist effects weaker than the endogenous full agonist (dopamine), specifically, aripiprazole's intrinsic agonist activity is so weak, that in nearly all real-life cases, aripiprazole will function solely as an antagonist at dopamine D2 receptors throughout the brain. [33][34][35][36]
Patent status
Otsuka's US patent on aripiprazole expires on October 20, 2014;[37] however, due to a pediatric extension, a generic will not become available until at least April 20, 2015.[3] Barr Laboratories (now Teva Pharmaceuticals) initiated a patent challenge under the Hatch-Waxman Act in March 2007.[38] As of 14 August 2009 (2009 -08-14)[update], this challenge is still in court.
Side effects
Akathisia[39], headache, unusual tiredness or weakness, nausea, vomiting, an uncomfortable feeling in the stomach, constipation, light-headedness, insomnia, sleepiness, shaking, and blurred vision.
Uncontrollable twitching or jerking movements, tremors, seizure, and weight gain. Some people may feel dizzy, especially when getting up from a lying or sitting position, or may experience a fast heart rate.
Neuroleptic malignant syndrome (Combination of fever, muscle stiffness, faster breathing, sweating, reduced consciousness, and sudden change in blood pressure and heart rate.)
Aripiprazole also causes sexual dysfunction.
Tardive dyskinesia (As with all antipsychotic medication, patients using aripiprazole may develop the permanent neurological disorder tardive dyskinesia.[40][41][42])
Stroke (While taking aripiprazole some elderly patients with dementia have suffered from stroke or 'mini' stroke.)
Other elderly patients may experience high blood sugar or the onset or worsening of diabetes.
Allergic reaction (such as swelling in the mouth or throat, itching, rash), increased production of saliva, speech disorder, nervousness, agitation, fainting, reports of abnormal liver test values, inflammation of the pancreas, muscle pain, weakness, stiffness, or cramps.
Overdosage
Children or adults who ingested acute overdoses have usually manifested central nervous system depression ranging from mild sedation to coma; serum concentrations of aripiprazole and dehydroaripiprazole in these patients were elevated by up to 3-4 fold over normal therapeutic levels, yet no deaths have yet been recorded.[43]
Drug interactions
Aripiprazole is a substrate of CYP2D6 and CYP3A4. Coadministration with medications that inhibit (e.g. paroxetine, fluoxetine) or induce (e.g. carbamazepine) these metabolic enzymes are known to increase and decrease, respectively, plasma levels of aripiprazole.[44] As such, anyone taking Abilify should be aware that their dosage of Abilify may need to be decreased.
Aripiprazole may change the subjective effects of alcohol. One study[45] found that aripiprazole increased the sedative effect and reduced the sense of euphoria normally associated with alcohol consumption. However, another alcohol study[46] found that there was no difference in subjective effect between a placebo group and a group taking aripiprazole.
Dosage forms
- Intramuscular injection, solution: 7.5 mg/mL (1.3 mL)
- Solution, oral: 1 mg/mL (150 mL) [contains propylene glycol, sucrose 400 mg/mL, and fructose 200 mg/mL; orange cream flavor]
- Tablet: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, 30 mg
- Tablet, orally disintegrating: 10 mg [contains phenylalanine 1.12 mg; creme de vanilla flavor]; 15 mg [contains phenylalanine 1.68 mg; creme de vanilla flavor]
Synthesis
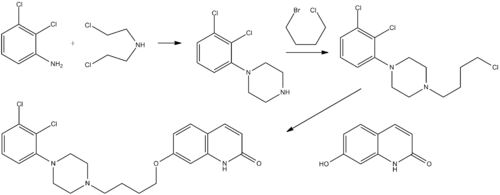
U.S. Patent 5,006,528
References
- ↑ Hitti, Miranda (20 November 2007). "FDA OKs Abilify for Depression". WebMD. http://www.webmd.com/depression/news/20071120/fda-oks-abilify-for-depression. Retrieved 8 December 2008.
- ↑ Stahl, Stephen M. 2006. Essential Psychopharmacology: The Prescriber's Guide. Cambridge University Press. New York, NY.
- ↑ 3.0 3.1 "Patent and Exclusivity Search Results". Electronic Orange Book. US Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021436&Product_No=001&table1=OB_Rx. Retrieved 8 December 2008.
- ↑ Keck PE, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, Ingenito G (2003). "A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania". Am J Psychiatry 160 (9): 1651–8. doi:10.1176/appi.ajp.160.9.1651. PMID 12944341. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=12944341.
- ↑ Sachs G, Sanchez R, Marcus R, Stock E, McQuade R, Carson W, Abou-Gharbia N, Impellizzeri C, Kaplita S, Rollin L, Iwamoto T (2006). "Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study". J. Psychopharmacol. (Oxford) 20 (4): 536–46. doi:10.1177/0269881106059693. PMID 16401666.
- ↑ Vieta E, T'joen C, McQuade RD, Carson WH, Marcus RN, Sanchez R, Owen R, Nameche L (2008). "Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study". Am J Psychiatry 165 (10): 1316–25. doi:10.1176/appi.ajp.2008.07101560. PMID 18381903.
- ↑ Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, McQuade RD, Carson WH (2009). "Aripiprazole monotherapy in the treatment of acute bipolar I mania: a randomized, double-blind, placebo- and lithium-controlled study". J Affect Disord 112 (1-3): 36–49. doi:10.1016/j.jad.2008.05.014. PMID 18835043.
- ↑ Keck PE, Calabrese JR, McIntyre RS, McQuade RD, Carson WH, Eudicone JM, Carlson BX, Marcus RN, Sanchez R (2007). "Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo". J Clin Psychiatry 68 (10): 1480–91. PMID 17960961.
- ↑ Keck PE, Calabrese JR, McQuade RD, Carson WH, Carlson BX, Rollin LM, Marcus RN, Sanchez R (2006). "A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder". J Clin Psychiatry 67 (4): 626–37. doi:10.4088/JCP.v67n0414. PMID 16669728.
- ↑ Young AH, Oren DA, Lowy A, McQuade RD, Marcus RN, Carson WH, Spiller NH, Torbeyns AF, Sanchez R (2009). "Aripiprazole monotherapy in acute mania: 12-week randomised placebo- and haloperidol-controlled study". Br J Psychiatry 194 (1): 40–8. doi:10.1192/bjp.bp.108.049965. PMID 19118324.
- ↑ Vieta E, Bourin M, Sanchez R, Marcus R, Stock E, McQuade R, Carson W, Abou-Gharbia N, Swanink R, Iwamoto T (2005). "Effectiveness of aripiprazole v. haloperidol in acute bipolar mania: double-blind, randomised, comparative 12-week trial". Br J Psychiatry 187: 235–42. doi:10.1192/bjp.187.3.235. PMID 16135860.
- ↑ Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P (2008). "Beneficial acute antidepressant effects of aripiprazole as an adjunctive treatment or monotherapy in bipolar patients unresponsive to mood stabilizers: results from a 16-week open-label trial". Expert Opin Pharmacother 9 (18): 3145–9. doi:10.1517/14656560802504490. PMID 19040335.
- ↑ Dunn RT, Stan VA, Chriki LS, Filkowski MM, Ghaemi SN (2008). "A prospective, open-label study of Aripiprazole mono- and adjunctive treatment in acute bipolar depression". J Affect Disord 110 (1-2): 70–4. doi:10.1016/j.jad.2008.01.004. PMID 18272230.
- ↑ Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P (2009). "Effect of aripiprazole on self-reported anhedonia in bipolar depressed patients". Psychiatry Res 165 (1-2): 193–6. doi:10.1016/j.psychres.2008.05.003. PMID 18973955.
- ↑ Thase ME, Jonas A, Khan A, Bowden CL, Wu X, McQuade RD, Carson WH, Marcus RN, Owen R (2008). "Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies". J Clin Psychopharmacol 28 (1): 13–20. doi:10.1097/jcp.0b013e3181618eb4. PMID 18204335.
- ↑ Muzina DJ, Momah C, Eudicone JM, Pikalov A, McQuade RD, Marcus RN, Sanchez R, Carlson BX (2008). "Aripiprazole monotherapy in patients with rapid-cycling bipolar I disorder: an analysis from a long-term, double-blind, placebo-controlled study". Int. J. Clin. Pract. 62 (5): 679–87. doi:10.1111/j.1742-1241.2008.01735.x. PMID 18373615.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021436s21,021713s16,021729s8,021866s8lbl.pdf Section 2.3 pp 7-8
- ↑ Yael Waknine (24 November 2009). "FDA Approves Aripiprazole to Treat Irritability in Autistic Children". Medscape Today. WebMD. http://www.medscape.com/viewarticle/713006. Retrieved 23 February 2010.
- ↑ 'Aripiprazole Blocks Reinstatement of Cocaine Seeking in an Animal Model of Relapse' Biological Psychiatry. Volume 61, Issue 5, Pages 582-590 (1 March 2007) http://www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223%2806%2900484-7/abstract
- ↑ Lawler CP et al. (1999). "Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes". Neuropsychopharmacology 20 (6): 612–27. doi:10.1016/S0893-133X(98)00099-2. PMID 10327430.
- ↑ Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR (2005). "Intrinsic Efficacy of Antipsychotics at Human D2, D3, and D4 Dopamine Receptors: Identification of the Clozapine Metabolite N-Desmethylclozapine as a D2/D3 Partial Agonist". J Pharmacol Exp Ther 315 (3): 1278–87. doi:10.1124/jpet.105.092155. PMID 16135699.
- ↑ Stark, AD et al. (2007). "Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT2A receptors: functional receptor-binding and in vivo electrophysiological studies". Psychopharmacology (Berl) 190 (3): 373–382. doi:10.1007/s00213-006-0621-y. PMID 17242925.
- ↑ Shapiro, DA et al. (2003). "Aripiprazole, A Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology". Neuropsychopharmacology 28 (8): 1400–1411. doi:10.1038/sj.npp.1300203. PMID 12784105.
- ↑ Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J (2006). "Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms". Biochem Pharmacol 71 (4): 521–9. doi:10.1016/j.bcp.2005.11.007. PMID 16336943.
- ↑ 25.0 25.1 "Abilify (Aripiprazole) - Clinical Pharmacology". DrugLib.com. 14 February 2007. http://www.druglib.com/druginfo/abilify/pharmacology/. Retrieved 8 December 2008.
- ↑ Kegeles, LS et al. (2008). "Dose–Occupancy Study of Striatal and Extrastriatal Dopamine D2 Receptors by Aripiprazole in Schizophrenia with PET and [18F]Fallypride". Neuropsychopharmacology 33 (13): 3111–3125. doi:10.1038/npp.2008.33. PMID 18418366.
- ↑ Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (2002). "Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride". Neuropsychopharmacology 27 (2): 248–59. doi:10.1016/S0893-133X(02)00304-4. PMID 12093598.
- ↑ "In This Issue". Am J Psychiatry 165 (8): A46. 2008. doi:10.1176/appi.ajp.2008.165.8.A46.
- ↑ 29.0 29.1 Hedlund PB (2009). "The 5-HT7 receptor and disorders of the nervous system: an overview". Psychopharmacology 206 (3): 345–54. doi:10.1007/s00213-009-1626-0. ISBN 0021300916260. PMID 19649616.
- ↑ 30.0 30.1 Sarkisyan G, Roberts AJ, Hedlund PB (2010). "The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior". Behavioural Brain Research 209 (1): 99–108. doi:10.1016/j.bbr.2010.01.022. PMID 20097233. PMC 2832919. http://linkinghub.elsevier.com/retrieve/pii/S0166-4328(10)00046-X.
- ↑ Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (2009). "Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo". Psychopharmacology 205 (1): 119–28. doi:10.1007/s00213-009-1521-8. ISBN 0021300915218. PMID 19337725.
- ↑ Caccia S (2007). "N-dealkylation of arylpiperazine derivatives: disposition and metabolism of the 1-aryl-piperazines formed". Current Drug Metabolism 8 (6): 612–22. doi:10.2174/138920007781368908. PMID 17691920. http://www.bentham-direct.org/pages/content.php?CDM/2007/00000008/00000006/0005F.SGM.
- ↑ "The temporal and extrastriatal D2/D3 receptor binding profile of aripiprazole in patients with schizophrenia -- Gründer et al. 48 (1002): 263P -- Society of Nuclear Medicine Annual Meeting Abstracts". http://jnumedmtg.snmjournals.org/cgi/content/meeting_abstract/48/MeetingAbstracts_2/263P-b. Retrieved 2010-05-30.
- ↑ Tadori Y, Miwa T, Tottori K, Burris KD, Stark A, Mori T, Kikuchi T (May 2005). "Aripiprazole's low intrinsic activities at human dopamine D2L and D2S receptors render it a unique antipsychotic". European Journal of Pharmacology 515 (1-3): 10–9. doi:10.1016/j.ejphar.2005.02.051. PMID 15894311.
- ↑ "www.psychiatrymmc.com". http://www.psychiatrymmc.com/displayArticle.cfm?articleID=article165. Retrieved 2010-05-30.
- ↑ "Aripiprazole: Dose-Response Relationship in Schizophrenia an... : CNS Drugs". http://adisonline.com/cnsdrugs/Abstract/2009/23090/Aripiprazole__Dose_Response_Relationship_in.5.aspx. Retrieved 2010-05-30.
- ↑ Carbostyril derivatives. October 20, 1989
- ↑ Barr Pharmaceuticals, Inc. (2007-03-20). "Barr Confirms Filing an Application with a Paragraph IV Certification for ABILIFY(R) Tablets". Press release. http://phx.corporate-ir.net/phoenix.zhtml?c=60908&p=irol-newsArticle&ID=975763&highlight=. Retrieved 2008-12-23.
- ↑ "Dose-dependent rapid-onset akathisia with aripiprazole in patients with schizoaffective disorder". http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2671776/. Retrieved 2010-08-07.
- ↑ Abbasian C, Power P (2009). "A case of aripiprazole and tardive dyskinesia". J Psychopharmacol (Oxford) 23 (2): 214–5. doi:10.1177/0269881108089591. PMID 18515468.
- ↑ Zaidi SH, Faruqui RA (2008). "Aripiprazole is associated with early onset of Tardive Dyskinesia like presentation in a patient with ABI and psychosis". Brain Inj 22 (1): 99–102. doi:10.1080/02699050701822493. PMID 18183513.
- ↑ Maytal G, Ostacher M, Stern TA (2006). "Aripiprazole-related tardive dyskinesia". CNS Spectr 11 (6): 435–9. PMID 16816781.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 105-106.
- ↑ "Abilify (Aripiprazole) - Warnings and Precautions". DrugLib.com. 14 February 2007. http://www.druglib.com/druginfo/abilify/warnings_precautions/. Retrieved 8 December 2008.
- ↑ Kranzler, Henry R. et al. (2008). "Effects of Aripiprazole on Subjective and Physiological Responses to Alcohol". Alcoholism: Clinical and Experimental Research 32 (4): 573–579. doi:10.1111/j.1530-0277.2007.00608.x. PMID 18261195.
- ↑ Konstantin Voronin, Patrick Randall, Hugh Myrick, Raymond Anton (2008). "Aripiprazole Effects on Alcohol Consumption and Subjective Reports in a Clinical Laboratory Paradigm—Possible Influence of Self-Control". Alcoholism: Clinical and Experimental Research 32 (11): 1954–1961. doi:10.1111/j.1530-0277.2008.00783.x. PMID 18782344.
External links
|
Antipsychotics (neuroleptics) (N05A) |
|
| Typical |
Benzamides: Levosulpiride • Nemonapride • Sulpiride • Sultopride • Tiapride • Veralipride;
Butyrophenones: Azaperone • Benperidol • Bromperidol • Droperidol • Fluanisone • Haloperidol • Lenperone • Moperone • Pipamperone • Spiperone • Timiperone • Trifluperidol;
Diphenylbutylpiperidines: Clopimozide • Fluspirilene • Penfluridol • Pimozide;
Phenothiazines: Acepromazine • Acetophenazine • Butaperazine • Carphenazine • Chloracizine • Chlorproethazine • Chlorpromazine • Cyamemazine • Dixyrazine • Fluacizine • Fluphenazine • Levomepromazine/Methotrimeprazine • Mesoridazine • Perazine • Periciazine • Perphenazine • Piperacetazine • Pipotiazine • Prochlorperazine • Promazine • Promethazine • Propiomazine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioproperazine • Thioridazine • Trifluoperazine • Triflupromazine;
Thioxanthenes: Chlorprothixene • Clopenthixol • Flupentixol • Thiothixene • Zuclopenthixol;
Tricyclics: Amoxapine • Butaclamol • Fluotracen • Loxapine • Metitepine/Methiothepin • Octoclothiepin • Trimipramine;
Others: Prothipendyl
|
|
| Atypical |
Benzamides: Amisulpride • Remoxipride;
Butyrophenones: Cinuperone • Setoperone;
Benzo(iso)oxazolepiperidines: Iloperidone • Ocaperidone • Paliperidone • Risperidone;
Benzo(iso)thiazolepiperazines: Lurasidone • Perospirone • Revospirone • Tiospirone • Ziprasidone;
Diphenylbutylpiperazines: Amperozide;
Phenylpiperazines: Aripiprazole • Bifeprunox • Elopiprazole • Umespirone;
Tricyclics: Asenapine • Carpipramine • Clocapramine • Clotiapine • Clozapine • Fluperlapine • Gevotroline • Metitepine/Methiothepin • Mosapramine • NDMC • Olanzapine • Piquindone • Quetiapine • Tenilapine • Zotepine;
Others: Blonanserin • Cariprazine • Molindone • Pimavanserin • Roxindole • Sarizotan • Sertindole • Spiramide
|
|
| Others |
Azacyclonol • Cannabidiol • D-Cycloserine • Lithium • Mifepristone • Oxypertine • Reserpine • Rimcazole • Secretin • Talnetant • Tetrabenazine • Vabicaserin |
|
#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Antidepressants (N06A) |
|
|
Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) |
|
|
|
|
|
|
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
|
|
|
|
Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs)
|
Brasofensine · BTS-74,398 · Cocaine · Diclofensine · DOV-21,947 · DOV-102,677 · DOV-216,303 · EXP-561 · Fezolamine · JNJ-7,925,476 · NS-2359 · PRC200-SS · Pridefrine · SEP-225,289 · SEP-227,162 · Tesofensine |
|
|
Norepinephrine reuptake inhibitors (NRIs)
|
Amedalin · Atomoxetine/Tomoxetine · Binedaline · Ciclazindol · Daledalin · Esreboxetine · Lortalamine · Mazindol · Nisoxetine · Reboxetine · Talopram · Talsupram · Tandamine · Viloxazine |
|
|
Dopamine reuptake inhibitors (DRIs)
|
Medifoxamine · Vanoxerine
|
|
|
Norepinephrine-dopamine reuptake inhibitors (NDRIs)
|
|
|
|
Norepinephrine-dopamine releasing agents (NDRAs)
|
|
|
|
Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)
|
4-Methyl-αMT · αET/Etryptamine · αMT/Metryptamine
|
|
|
Selective serotonin reuptake enhancers (SSREs)
|
Tianeptine
|
|
|
Others
|
|
|
|
|
|
Receptor antagonists and/or reuptake inhibitors |
|
|
Serotonin antagonists and reuptake inhibitors (SARIs)
|
|
|
|
Noradrenergic and specific serotonergic antidepressants (NaSSAs)
|
Aptazapine · Esmirtazapine · Mianserin · Mirtazapine · Setiptiline/Teciptiline |
|
|
Norepinephrine-dopamine disinhibitors (NDDIs)
|
Agomelatine
|
|
|
Serotonin modulators and stimulators (SMSs)
|
Lu AA21004
|
|
|
|
|
Tricyclic and tetracyclic antidepressants (TCAs/TeCAs) |
|
Tricyclics: Amezepine · Amineptine · Amitriptyline · Amitriptylinoxide · Azepindole · Butriptyline · Cianopramine · Clomipramine · Cotriptyline · Cyanodothiepin · Demexiptiline · Depramine/Balipramine · Desipramine · Dibenzepine · Dimetacrine · Dosulepin/Dothiepin · Doxepin · Enprazepine · Fluotracen · Hepzidine · Homopipramol · Imipramine · Imipraminoxide · Intriptyline · Iprindole · Ketipramine · Litracen · Lofepramine · Losindole · Mariptiline · Melitracen · Metapramine · Mezepine · Naranol · Nitroxazepine · Nortriptyline · Noxiptiline · Octriptyline · Opipramol · Pipofezine · Propizepine · Protriptyline · Quinupramine · Tampramine · Tianeptine · Tienopramine · Trimipramine; Tetracyclics: 7-OH-Amoxapine · Amoxapine · Aptazapine · Azipramine · Ciclazindol · Ciclopramine · Esmirtazapine · Loxapine · Maprotiline · Mazindol · Mianserin · Mirtazapine · Oxaprotiline · Setiptiline/Teciptiline
|
|
|
|
Monoamine oxidase inhibitors (MAOIs) |
|
Nonselective: Irreversible: Benmoxin · Echinopsidine · Iproclozide · Iproniazid · Isocarboxazid · Mebanazine · Metfendrazine · Nialamide · Octamoxin · Phenelzine · Pheniprazine · Phenoxypropazine · Pivalylbenzhydrazine · Safrazine · Tranylcypromine; Reversible: Caroxazone · Paraxazone; MAOA-Selective: Irreversible: Clorgyline; Reversible: Amiflamine · Bazinaprine · Befloxatone · Befol · Brofaromine · Cimoxatone · Esuperone · Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) · Methylene Blue · Metralindole · Minaprine · Moclobemide · Pirlindole · Sercloremine · Tetrindole · Toloxatone · Tyrima; MAOB-Selective: Irreversible: Ladostigil · Mofegiline · Pargyline · Rasagiline · Selegiline; Reversible: Lazabemide · Milacemide
|
|
|
|
Azapirones and other 5-HT1A receptor agonists |
|
Alnespirone · Aripiprazole · Befiradol · Buspirone · Eptapirone · Flesinoxan · Flibanserin · Gepirone · Ipsapirone · Oxaflozane · Tandospirone · Vilazodone · Zalospirone |
|
|
|
|
|
|
Research compounds and miscellaneous agents |
|
|
5-HT4R agonists
|
RS-67,333 · SL65.0155
|
|
|
5-HT7R antagonists
|
Amisulpride
|
|
|
|
|
|
|
β3-Adrenoceptor agonists
|
Amibegron · Solabegron
|
|
|
|
|
|
|
|
|
|
|
COMT inhibitors
|
Entacapone · Tolcapone
|
|
|
CRF1R antagonists
|
Antalarmin · CP-154,526 · Pexacerfont · Pivagabine
|
|
|
D2/D3AR antagonists
|
Amisulpride · Sulpiride
|
|
|
D2/D3/D4R agonists
|
Piribedil · Pramipexole · Ropinirole · Rotigotine · Roxindole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Agomelatine · Melatonin · Ramelteon · Tasimelteon |
|
|
NK1R antagonists
|
Aprepitant · Casopitant · Fosaprepitant · L-733,060 · Maropitant · Vestipitant
|
|
|
|
|
|
|
PDE4 inhibitors
|
Mesembrine (Kanna) · Rolipram
|
|
|
|
|
|
|
|
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Adrenergics |
|
|
Receptor ligands |
|
|
α1
|
Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.
|
|
|
α2
|
Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.
|
|
|
|
Agonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • N-Isopropyloctopamine • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • Xipranolol
|
|
|
|
|
Reuptake inhibitors |
|
|
NET
|
Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tramadol • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine ( Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH
|
3,4-Dihydroxystyrene
|
|
|
TH
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
DBH
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
PNMT
|
CGS-19281A • SKF-64139 • SKF-7698
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.
|
|
|
COMT
|
Entacapone • Tolcapone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Dopaminergics |
|
|
Receptor ligands |
|
|
Agonists
|
Adamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635
|
|
|
Antagonists
|
Typical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Ocaperidone • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,566 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • Yohimbine
|
|
|
|
|
Reuptake inhibitors |
|
|
|
|
DAT inhibitors
|
Piperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane ( 123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tripelennamine
|
|
|
|
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH inhibitors
|
3,4-Dihydroxystyrene
|
|
|
TH inhibitors
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD / DDC inhibitors
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
|
|
|
COMT inhibitors
|
Entacapone • Tolcapone
|
|
|
DBH inhibitors
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity Enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Histaminergics |
|
Receptor
ligands |
|
H1
|
Agonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorpheniramine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; 3rd generation: Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic Antidepressants ( Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic Antidepressants (Mianserin, Mirtazapine, etc) • Serotonin Antagonists and Reuptake Inhibitors ( Trazodone, Nefazodone) • Typical Antipsychotics ( Chlorpromazine, Thioridazine, etc) • Atypical Antipsychotics ( Clozapine, Olanzapine, Quetiapine, etc)
|
|
|
H2
|
Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Bisfentidine • Burimamide • Cimetidine • Dalcotidine • Donetidine • Ebrotidine • Etintidine • Famotidine • Lafutidine • Lamtidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Mifentidine • Niperotidine • Nizatidine • Osutidine • Oxmetidine • Pibutidine • Quisultidine/Quisultazine • Ramixotidine • Ranitidine • Roxatidine • Sufotidine • Tiotidine • Tuvatidine • Venritidine • Zaltidine
|
|
|
H3
|
Agonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • SCH-79,687 • Thioperamide • Tiprolisant • VUF-5,681
|
|
|
H4
|
Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002
|
|
|
Reuptake
inhibitors |
|
|
Enzyme
inhibitors |
|
|
|
HDC inhibitors
|
α-FMH • Brocresine • Catechin • Cyanidanol-3 • McN-A-1293 • ME • Meciadanol • Naringenin • Thiazol-4-yimethoxyamine • Tritoqualine • Zy-15,029
|
|
|
|
|
|
HNMT inhibitors
|
Amodiaquine • BW-301U • Diphenhydramine • Harmaline • Metoprine • Quinacrine • SKF-91,488 • Tacrine |
|
|
DAO inhibitors
|
1,4-Diamino-2-butyne • Aminoguanidine
|
|
|
|
| Others |
|
|
|
Serotonergics |
|
|
5-HT1 receptor ligands |
|
|
5-HT1A
|
Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • Lu AA21004 • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92016A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • Xylamidine
|
|
|
5-HT1B
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • Yohimbine
|
|
|
5-HT1D
|
Agonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • Ziprasidone
|
|
|
5-HT1E
|
Agonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/Methiothepin
|
|
|
5-HT1F
|
Agonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin
|
|
|
|
|
5-HT2 receptor ligands |
|
|
|
5-HT2A
|
Agonists: Lysergamides: ALD-52 • Ergonovine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Methysergide; Phenethylamines: 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • Yohimbine
|
|
|
5-HT2B
|
Agonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • Yohimbine
|
|
|
5-HT2C
|
Agonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Lu AA24530 • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine
|
|
|
|
|
5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands |
|
|
|
5-HT3
|
Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • Lu AA21004 • Lu AA24530 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Thujone • Xenon
|
|
|
5-HT4
|
Agonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Zacopride; Others: 5-MT • BIMU-8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • TD-5108
Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186
|
|
|
5-HT5A
|
Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.
|
|
|
5-HT6
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • N-Methyl-5-HT • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • EGIS-12233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro 04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457
|
|
|
5-HT7
|
Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507
|
|
|
|
|
Reuptake inhibitors |
|
|
SERT
|
Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lu AA21004 • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Vilazodone • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorpheniramine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Meperidine (Pethidine) • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefrine • Roxindole • SB-649,915 • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Aminoindanes: 5-IAI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Diethylcathinone • Dimethylcathinone • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • NAP • Norfenfluramine • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pFPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • Viqualine
|
|
|
|
Enzyme inhibitors |
|
|
|
|
TPH
|
AGN-2979 • Fenclonine
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Reuptake enhancers: Tianeptine
|
|
|
|
|
Piperazines |
|
Simple piperazines
(no additional rings) |
1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPES
|
|
| Phenylpiperazines |
Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635 |
|
| Benzylpiperazines |
2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • Vesnarinone |
|
Diphenylalkylpiperazines
(benzhydrylalkylpiperazines) |
Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • Vanoxerine |
|
| Pyrimidinylpiperazines |
Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • Zalospirone
|
|
| Pyridinylpiperazines |
Atevirdine • Azaperone • Pyridinylpiperazine
|
|
| Benzo(iso)thiazolylpiperazines |
Lurasidone • Perospirone • Revospirone • Tiospirone • Ziprasidone
|
|
Tricyclics
(piperazine attached via side chain) |
Amoxapine • Clopenthixol • Clozapine • Flupentixol • Fluphenazine • Loxapine • Olanzapine • Opipramol • Perazine • Perphenazine • Pirenzepine • Prochlorperazine • Thiethylperazine • Thiothixene • Trifluoperazine • Zuclopenthixol |
|
| Others |
6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • Zipeprol |
|