Fermium
Not to be confused with "Ferrum", the Latin name for
Iron.
|
|
| Appearance |
| unknown, probably silvery, white or metallic gray |
| General properties |
| Name, symbol, number |
fermium, Fm, 100 |
| Pronunciation |
 /ˈfɜrmiəm/ /ˈfɜrmiəm/
FER-mee-əm |
| Element category |
actinide |
| Group, period, block |
n/a, 7, f |
| Standard atomic weight |
(257)g·mol−1 |
| Electron configuration |
[Rn] 5f12 7s2 |
| Electrons per shell |
2, 8, 18, 32, 30, 8, 2 (Image) |
| Physical properties |
| Phase |
solid |
| Melting point |
1800 K, 1527 °C, 2781 °F |
| Atomic properties |
| Oxidation states |
2, 3 |
| Electronegativity |
1.3 (Pauling scale) |
| Ionization energies |
1st: 627 kJ·mol−1 |
| Miscellanea |
| CAS registry number |
7440-72-4 |
| Most stable isotopes |
| Main article: Isotopes of fermium |
| iso |
NA |
half-life |
DM |
DE (MeV) |
DP |
| 252Fm |
syn |
25.39 h |
SF |
- |
- |
| α |
7.153 |
248Cf |
| 253Fm |
syn |
3 d |
ε |
0.333 |
253Es |
| α |
7.197 |
249Cf |
| 255Fm |
syn |
20.07 h |
SF |
- |
- |
| α |
7.241 |
251Cf |
| 257Fm |
syn |
100.5 d |
α |
6.864 |
253Cf |
| SF |
- |
- |
|
|
Fermium (pronounced /ˈfɜrmiəm/, FER-mee-əm) is a synthetic element with the symbol Fm and atomic number 100. A highly radioactive metallic transuranic element of the actinide series, fermium is made by bombarding plutonium with neutrons and is named after nuclear physicist Enrico Fermi. Fermium is the eighth transuranic element.
Characteristics
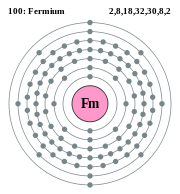
Electron shell diagram of fermium
Only small amounts of fermium have been produced or isolated. Thus, relatively little is known about its chemical properties. Only the +3 oxidation state of the element appears to exist in aqueous solution. 254Fm and heavier isotopes can be synthesized by intense neutron bombardment of lighter elements (especially uranium and plutonium). During this, successive neutron captures mixed with beta decays build the fermium isotope. The intense neutron bombardment conditions needed to create fermium exist in thermonuclear explosions and can be replicated in the laboratory (such as in the High Flux Isotope Reactor at Oak Ridge National Laboratory). The synthesis of element 102 (nobelium) was confirmed when 250Fm was chemically identified. Like all synthetic elements, it is extremely radioactive and highly toxic.
History
Fermium, named for the Italian-American physicist Enrico Fermi, was first discovered by a team led by Albert Ghiorso in 1952. The team found 255Fm in the collected dust from the first hydrogen bomb explosion on November 1, 1952. See the Ivy Mike H-bomb test. This isotope was created when U-238 combined with 17 neutrons during the intense temperatures of the nuclear test explosion. Eight beta decays also occurred to create fermium nuclei. This work was overseen by the University of California Radiation Laboratory, the Argonne National Laboratory, and the Los Alamos Scientific Laboratory. All these findings were kept secret until 1955 due to Cold War tensions.[1] Samples of sea coral impacted from the first thermonuclear explosion of November 1952 were also used.[2]
In late 1953 and early 1954 a team from the Nobel Institute for Physics in Stockholm, Sweden, bombarded a uranium-238 target with oxygen-16 ions, producing an alpha-emitting element with an atomic mass of about 250 and with 100 protons (in other words, element 250Unn).[3] The Nobel Insitute's team did not claim this discovery until 1954. The isotope that they produced was later positively identified as 250Fm.
Isotopes
17 radioisotopes of fermium have been characterized, with the most stable being 257Fm with a half-life of about 100 days, 253Fm with a half-life of three days, 252Fm with a half-life of 25.4 hours, and 255Fm with a half-life of 20.1 hours. All of the remaining radioactive isotopes have half-lives that are less than 5.4 hours, and the majority of these have half-lives that are less than three minutes. Fermium also has one known metastable state, 250mFm (t½ 1.8 seconds). The isotopes of fermium range in atomic mass from 242.073 atomic mass units (242Fm) to 259.101 a.m.u. (259Fm).
References
- ↑ Ghiorso, A.; Thompson, S. G.; Higgins, G. H. ; Seaborg, G. T.; Studier, M. H.; Fields, P. R.; Fried, S. M.; Diamond, H.; Mech, J. F.; Pyle, G. L.; Huizenga, J. R.; Hirsch, A.; Manning, W. M.; Browne, C. I.; Smith, H. L.; Spence, R. W. (1955). "New Elements Einsteinium and Fermium, Atomic Numbers 99 and 100". Physical Review 99: 1048–1049. doi:10.1103/PhysRev.99.1048. http://prola.aps.org/abstract/PR/v99/i3/p1048_1.
- ↑ Albert Ghiorso (2003). "Einsteinium and Fermium". Chemical and Engineering News. http://pubs.acs.org/cen/80th/einsteiniumfermium.html.
- ↑ Atterling, Hugo; Forsling, Wilhelm; Holm, Lennart W.; Melander, Lars; Åström, Björn (1954). "Element 100 Produced by Means of Cyclotron-Accelerated Oxygen Ions". Physical Review 95: 585–586. doi:10.1103/PhysRev.95.585.2.
External links
