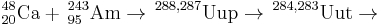Ununtrium
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unknown | |||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | ununtrium, Uut, 113 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | oon-OON-tree-əm |
||||||||||||||||||||||||||||||||||||||||||||||||
| Category notes | presumably other metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 13, 7, p | ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [286]g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p1 (guess based on thallium) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 3 (Image) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54084-70-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of ununtrium | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Ununtrium (pronounced /uːnˈuːntri.əm/ (![]() listen)[1] oon-OON-tree-əm) is the temporary name of a synthetic element with the temporary symbol Uut and atomic number 113.
listen)[1] oon-OON-tree-əm) is the temporary name of a synthetic element with the temporary symbol Uut and atomic number 113.
It is placed as the heaviest member of the group 13 (IIIA) elements although a sufficiently stable isotope is not known at this time that would allow chemical experiments to confirm its position. It was first detected in 2003 in the decay of ununpentium and was synthesized directly in 2004. Only fourteen atoms of ununtrium have been observed to date. The longest-lived isotope known is 286Uut with a half-life of ~20 s, allowing first chemical experiments to study its chemistry.
Contents |
History
Discovery profile
The first report of ununtrium was in August 2003 when it was identified as a decay product of ununpentium. These results were published on February 1, 2004, by a team composed of Russian scientists at Dubna (Joint Institute for Nuclear Research), and American scientists at the Lawrence Livermore National Laboratory.[2][3]
On July 23, 2004, a team of Japanese scientists at RIKEN detected a single atom of 278Uut using the cold fusion reaction between bismuth-209 and zinc-70. They published their results on September 28, 2004.[4]
Support for their claim appeared in 2004 when scientists at the Institute of Modern Physics (IMP) identified 266Bh as decaying with identical properties to their single event (see bohrium).
The RIKEN team produced a further atom on April 2, 2005, although the decay data was different from the first chain, and may be due to the formation of a meta-stable isomer.
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununtrium by conducting chemical experiments on the decay daughter 268Db. In experiments in Jun 2004 and Dec 2005, the Dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities. Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 and Z=113 to the parent and daughter nuclei.[5][6]
Theoretical estimates of alpha-decay half-lives of alpha-decay chains from element 113 are in good agreement with the experimental data.[7]
Naming
The element with atomic number 113 is historically known as eka-thallium. Ununtrium (Uut) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 113 (or E113).
Proposed names by claimants
Claims to the discovery of ununtrium have been put forward by Dmitriev of the Dubna team and Morita of the RIKEN team. The IUPAC/IUPAP Joint Working Party will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. The table below gives the names that the teams above have suggested and which can be verified by press interviews.
| Group | Proposed Name | Derivation |
|---|---|---|
| RIKEN | Japonium[8] | Japan: country of group claimants |
| RIKEN | Rikenium[8] | RIKEN: institute of group claimants |
Isotopes and nuclear properties
Nucleosynthesis
Target-Projectile combinations leading to Z=113 compound nuclei
The below table contains various combinations of targets and projectiles (both at max no. of neutrons) which could be used to form compound nuclei with an atomic number of 113.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 71Ga | 279Uut | Reaction yet to be attempted |
| 209Bi | 70Zn | 279Uut | Successful reaction |
| 232Th | 51V | 283Uut | Reaction yet to be attempted |
| 238U | 45Sc | 283Uut | Reaction yet to be attempted |
| 237Np | 48Ca | 285Uut | Successful reaction |
| 244Pu | 41K | 285Uut | Reaction yet to be attempted |
| 243Am | 40Ar | 283Uut | Reaction yet to be attempted |
| 248Cm | 37Cl | 285Uut | Reaction yet to be attempted |
| 249Bk | 36S | 285Uut | Reaction yet to be attempted |
| 249Cf | 31P | 280Uut | Reaction yet to be attempted |
Cold fusion
This section deals with the synthesis of nuclei of ununtrium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10-20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.
209Bi(70Zn,xn)279-xUut (x=1)
The synthesis of ununtrium was first attempted in 1998 by the team at GSI using the above cold fusion reaction. In two separate runs, they were unable to detect any atoms and calculated a cross section limit of 900 fb.[9] They repeated the experiment in 2003 and lowered the limit further to 400 fb.[9] In late 2003, the emerging team at RIKEN using their efficient apparatus GARIS attempted the reaction and reached a limit of 140 fb. In December 2003-August 2004, they resorted to 'brute force' and performed an eight-month-long irradiation in which they increased the sensitivity to 51 fb. They were able to detect a single atom of 278Uut.[4] They repeated the reaction in several runs in 2005 and were able to synthesize a second atom. They calculated a record-low 31 fb for the cross section for the 2 atoms. The reaction was repeated again in 2006 with two long production runs but no further atoms were detected. This lowered the yield further to the current value of just 23 fb.
Hot fusion
This section deals with the synthesis of nuclei of ununtrium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40-50 MeV, hence "hot"), leading to a reduced probability of survival from fission. The excited nucleus then decays to the ground state via the emission of 3-5 neutrons. Fusion reactions utilizing 48Ca nuclei usually produce compound nuclei with intermediate excitation energies (~30-35 MeV) and are sometimes referred to as "warm" fusion reactions. This leads, in part, to relatively high yields from these reactions.
237Np(48Ca,xn)285-xUut (x=3)
In June 2006, the Dubna-Livermore team synthesised ununtrium directly in the "warm" fusion reaction between neptunium-237 and calcium-48 nuclei. Two atoms of 282Uut were detected with a cross section of 900 fb.[10]
As a decay product
Ununtrium has also been detected in the decay of ununpentium and ununseptium.
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 278Uut | 2004 | 209Bi(70Zn,n) [4] |
| 279Uut | unknown | |
| 280Uut | unknown | |
| 281Uut | unknown | |
| 282Uut | 2006 | 237Np(48Ca,3n)[10] |
| 283Uut | 2003 | 243Am(48Ca,4n)[2] |
| 284Uut | 2003 | 243Am(48Ca,3n)[2] |
| 285Uut | 2009 | 249Bk(48Ca,4n) |
| 286Uut | 2009 | 249Bk(48Ca,3n) |
Yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing ununtrium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 70Zn | 209Bi | 279Uut | 23 fb |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununtrium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 3n | 4n | 5n |
|---|---|---|---|---|---|
| 48Ca | 237Np | 285Uut | 0.9 pb, 39.1 MeV [10] |
Theoretical calculations
Evaporation residue cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
DNS = Di-nuclear system; σ = cross section
| Target | Projectile | CN | Channel (product) | σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 209Bi | 70Zn | 279Uut | 1n (278113) | 30 fb | DNS | [11] |
| 237Np | 48Ca | 285Uut | 3n (282113) | 0.4 pb | DNS | [12] |
Chemical properties
Extrapolated chemical properties
Oxidation states
Ununtrium is projected to be the first member of the 7p series of elements and the heaviest member of group 13 (IIIA) in the Periodic Table, below thallium. Each of the members of this group show the group oxidation state of +III. However, thallium has a tendency to form only a stable +I state due to the "inert pair effect", explained by the relativistic stabilisation of the 7s-orbitals, resulting in a higher ionisation potential and weaker tendency to participate in bonding.
Chemistry
Ununtrium should portray eka-thallium chemical properties and should therefore form a monoxide, Uut2O, and monohalides, UutF, UutCl, UutBr, and UutI. If the +III state is accessible, it is likely that it is only possible in the oxide, Uut2O3, and fluoride, UutF3.
See also
- Isotopes of ununtrium
- Island of stability
References
- ↑ J. Chatt (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51: 381–384. doi:10.1351/pac197951020381.
- ↑ 2.0 2.1 2.2 "Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291-x115", Oganessian et al., JINR Preprints, 2003. Retrieved on 3 March 2008
- ↑ Oganessian, Yu. Ts. (2004). "Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291-x115". Physical Review C 69: 021601. doi:10.1103/PhysRevC.69.021601.
- ↑ 4.0 4.1 4.2 Morita, Kosuke (2004). "Experiment on the Synthesis of Element 113 in the Reaction 209Bi(70Zn, n)278113". Journal of the Physical Society of Japan 73: 2593. doi:10.1143/JPSJ.73.2593.
- ↑ "Results of the experiment on chemical identification of Db as a decay product of element 115", Oganessian et al., JINR preprints, 2004. Retrieved on 3 March 2008
- ↑ Oganessian, Yu. Ts. (2005). "Synthesis of elements 115 and 113 in the reaction 243Am + 48Ca". Physical Review C 72: 034611. doi:10.1103/PhysRevC.72.034611.
- ↑ P. Roy Chowdhury, D. N. Basu and C. Samanta (2007). "α decay chains from element 113". Phys. Rev. C 75: 047306. doi:10.1103/PhysRevC.75.047306.
- ↑ 8.0 8.1 "RIKEN NEWS November 2004". http://www.riken.go.jp/engn/r-world/info/release/news/2004/nov/index.html. Retrieved 9 February 2008.
- ↑ 9.0 9.1 "Search for element 113", Hofmann et al., GSI report 2003. Retrieved on 3 March 2008
- ↑ 10.0 10.1 10.2 Oganessian et al. (2007). "Synthesis of the isotope 282113 in the 237Np+48Ca fusion reaction". Phys. Rev. C 76: 011601(R). doi:10.1103/PhysRevC.76.011601. http://nrv.jinr.ru/pdf_file/PhysRevC_76_011601.pdf.
- ↑ Feng, Zhao-Qing (2007). "Formation of superheavy nuclei in cold fusion reactions". Physical Review C 76: 044606. doi:10.1103/PhysRevC.76.044606. http://arxiv.org/pdf/0707.2588.
- ↑ Feng, Z (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A 816: 33. doi:10.1016/j.nuclphysa.2008.11.003. http://arxiv.org/pdf/0803.1117.
External links
- WebElements.com: Ununtrium
- Uut and Uup Add Their Atomic Mass to Periodic Table
- Apsidium: Ununtrium 113 Uut
- Discovery of Elements 113 and 115
- Superheavy elements
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||