Rhenium
|
||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
grayish white |
||||||||||||||||||||||
| General properties | ||||||||||||||||||||||
| Name, symbol, number | rhenium, Re, 75 | |||||||||||||||||||||
| Pronunciation | /ˈriːniəm/ REE-nee-əm | |||||||||||||||||||||
| Element category | transition metal | |||||||||||||||||||||
| Group, period, block | 7, 6, d | |||||||||||||||||||||
| Standard atomic weight | 186.207g·mol−1 | |||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d5 6s2 | |||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 13, 2 (Image) | |||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||
| Density (near r.t.) | 21.02 g·cm−3 | |||||||||||||||||||||
| Liquid density at m.p. | 18.9 g·cm−3 | |||||||||||||||||||||
| Melting point | 3459 K, 3186 °C, 5767 °F | |||||||||||||||||||||
| Boiling point | 5869 K, 5596 °C, 10105 °F | |||||||||||||||||||||
| Heat of fusion | 60.43 kJ·mol−1 | |||||||||||||||||||||
| Heat of vaporization | 704 kJ·mol−1 | |||||||||||||||||||||
| Specific heat capacity | (25 °C) 25.48 J·mol−1·K−1 | |||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||
| Oxidation states | 7, 6, 5, 4, 3, 2, 1, 0, -1 (mildly acidic oxide) |
|||||||||||||||||||||
| Electronegativity | 1.9 (Pauling scale) | |||||||||||||||||||||
| Ionization energies (more) |
1st: 760 kJ·mol−1 | |||||||||||||||||||||
| 2nd: 1260 kJ·mol−1 | ||||||||||||||||||||||
| 3rd: 2510 kJ·mol−1 | ||||||||||||||||||||||
| Atomic radius | 137 pm | |||||||||||||||||||||
| Covalent radius | 151±7 pm | |||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||
| Magnetic ordering | paramagnetic[1] | |||||||||||||||||||||
| Electrical resistivity | (20 °C) 193 nΩ·m | |||||||||||||||||||||
| Thermal conductivity | (300 K) 48.0 W·m−1·K−1 | |||||||||||||||||||||
| Thermal expansion | 6.2 µm/(m·K) | |||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 4700 m/s | |||||||||||||||||||||
| Young's modulus | 463 GPa | |||||||||||||||||||||
| Shear modulus | 178 GPa | |||||||||||||||||||||
| Bulk modulus | 370 GPa | |||||||||||||||||||||
| Poisson ratio | 0.30 | |||||||||||||||||||||
| Mohs hardness | 7.0 | |||||||||||||||||||||
| Vickers hardness | 2450 MPa | |||||||||||||||||||||
| Brinell hardness | 1320 MPa | |||||||||||||||||||||
| CAS registry number | 7440-15-5 | |||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||
| Main article: Isotopes of rhenium | ||||||||||||||||||||||
|
||||||||||||||||||||||
Rhenium (pronounced /ˈriːniəm/, REE-nee-əm) is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. The free element has the third-highest melting point of any element, exceeded only by tungsten and carbon. Rhenium resembles manganese chemically and is obtained as a by-product of molybdenum and copper refinement. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7.
Discovered in 1925, rhenium was the last naturally occurring stable element to be discovered (francium was the last identified naturally occurring element, but it is unstable). Rhenium was named after the river Rhine.
Nickel-based superalloys for use in jet engines contain up to 6% of rhenium, making jet engine construction the largest use for the element, with chemical industry catalytic uses being next-most important. Because of the low availability relative to demand, rhenium is among the most expensive industrial metals, with an average price exceeding US$6,000 per kilogram, as of 2009.
Contents |
History
Rhenium (Latin Rhenus meaning Rhine)[2] was the next-to-last naturally occurring element to be discovered and the last element to be discovered having a stable isotope.[3] The existence of a yet undiscovered element at this position in the periodic table had been predicted by Henry Moseley in 1914.[4] It is generally considered to have been discovered by Walter Noddack, Ida Tacke, and Otto Berg in Germany. In 1925 they reported that they detected the element in platinum ore and in the mineral columbite. They also found rhenium in gadolinite and molybdenite.[5] In 1928 they were able to extract 1 g of the element by processing 660 kg of molybdenite.[6] The process was so complicated and expensive that production was discontinued until early 1950 when tungsten-rhenium and molybdenum-rhenium alloys were prepared. These alloys found important applications in industry that resulted in a great demand for the rhenium produced from the molybdenite fraction of porphyry copper ores.
In 1908, Japanese chemist Masataka Ogawa announced that he discovered the 43rd element and named it nipponium (Np) after Japan (which is Nippon in Japanese). However, later analysis indicated the presence of rhenium (element 75), not element 43.[7] The symbol Np was later used for the element neptunium.
Characteristics
Rhenium is a silvery-white metal with one of the highest melting points of all elements, exceeded by only tungsten and carbon. It is also one of the densest, exceeded only by platinum, iridium and osmium.
Its usual commercial form is a powder, but this element can be consolidated by pressing and sintering in a vacuum or hydrogen atmosphere. This procedure yields a compact solid having a density above 90% of the density of the metal. When annealed this metal is very ductile and can be bent, coiled, or rolled.[8] Rhenium-molybdenum alloys are superconductive at 10 K; tungsten-rhenium alloys are also superconductive[9] around 4-8 K, depending on the alloy. Rhenium metal superconducts at 2.4 K.[10][11]
Isotopes
Rhenium has a stable isotope, rhenium-185, which nevertheless occurs in minority abundance, a situation found only in one other element (indium). Naturally occurring rhenium is 37.4% 185Re, which is stable, and 62.6% 187Re, which is unstable but has a very long half-life (~1010 years). This lifetime is affected by the charge state of rhenium atom.[12][13] The beta decay of 187Re is used for rhenium-osmium dating of ores. The available energy for this beta decay (2.6 keV) is one of the lowest known among all radionuclides. There are twenty-six other recognized radioactive isotopes of rhenium.[14]
Compounds
See also Category: Rhenium compounds
Rhenium has nine known oxidation states: −1, 0, +1, +2, +3, +4, +5, +6 and +7.[15] The oxidation states +7, +6, +4, and +2 are the most common.[15]
The most common rhenium compounds are the oxides and the halides exhibiting a broad oxidation number spectrum: Re2O7, ReO3, Re2O5, ReO2, and Re2O3 are the known oxides, and ReF7, ReCl6, ReCl5, ReCl4 and ReCl3 are a few of the known halogen derivatives.[16] Known sulfides are ReS2 and Re2S7.[16]
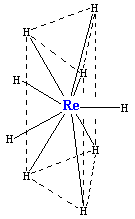
Reaction of rhenium with hydrogen produces the negatively charged hydride [ReH9]2− ion, which is isostructural with [TcH9]2−. It consists of a trigonal prism with Re atom in the center and six hydrogen atoms at the corners. Three more hydrogens make a triangle lying parallel to the base and crossing the prism in its center (see figure). Although those hydrogen atoms are not equivalent geometrically, their electronic structure is almost the same. The coordination number 9 in this complex is the highest for a rhenium complex. Two protons in it can be replaced by sodium (Na+) or potassium (K+) ions.[17]
Rhenium is most available commercially as the sodium and ammonium perrhenates. It is also readily available as dirhenium decacarbonyl; these three compounds are common entry points to rhenium chemistry. Various perrhenate salts may be easily converted to tetrathioperrhenate by the action of ammonium hydrosulfide.[18] It is possible to reduce the dirhenium decacarbonyl Re2(CO)10 by reacting it with sodium amalgam to Na[Re(CO)5] with rhenium in the formal oxidation state −1.[19] Dirhenium decacarbonyl may be oxidatively cleaved with bromine to give bromopentacarbonylrhenium(I),[20] then reduced with zinc and acetic acid to pentacarbonylhydridorhenium:[21]
- Re2(CO)10 + Br2 → Re(CO)5Br
- Re(CO)5Br + Zn + HOAc → Re(CO)5H + ZnBr(OAc)
Bromopentacarbonylrhenium(I) may be decarbonylated to give the rhenium tricarbonyl fragment either by refluxing in water:[22]
- Re(CO)5Br + 3 H2O → [Re(CO)3(H2O)3]Br + 2 CO
or by reacting with tetraethylammonium bromide:[23]
- Re(CO)5Br + 2 NEt4Br → [NEt4]2[Re(CO)3Br3] + 2 CO
Rhenium diboride (ReB2) is a hard compound having the hardness similar to that of tungsten carbide, silicon carbide, titanium diboride or zirconium diboride.[24]
Rhenium was originally thought to form the rhenide anion, Re−, in which it has the −1 oxidation state. This was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate (KReO4) by potassium metal.[25] "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula KRe·4H2O.[26] This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rhenide" ion is actually a hydridorhenate complex. "Potassium rhenide" was shown to be in fact the nonahydridorhenate, K2ReH9, containing the ReH92− anion in which the oxidation state of rhenium is actually +7.[27][28] Other methods of reduction of perrhenate salts yield compounds containing other hydrido- complexes, including ReH3(OH)3(H2O)−.[29]
Occurrence

Rhenium is one of the rarest elements in Earth's crust with an average concentration of 1 ppb;[16] other sources quote the number of 0.5 ppb making it the 77th most abundant element in Earth's crust.[30] Rhenium is probably not found free in nature (its possible natural occurrence is uncertain), but occurs in amounts up to 0.2%[16] in the mineral molybdenite (which is primarily molybdenum disulfide), the major commercial source, although single molybdenite samples with up to 1.88% have been found.[31] Chile has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005.[32] It was only recently that the first rhenium mineral was found and described (in 1994), a rhenium sulfide mineral (ReS2) condensing from a fumarole on Russia's Kudriavy volcano, Iturup island, in the Kurile Islands.[33] Named rheniite, this rare mineral commands high prices among collectors,[34] but is not an economically viable source of the element.
Production

Commercial rhenium is extracted from molybdenum roaster-flue gas obtained from copper-sulfide ores. Some molybdenum ores contain 0.001% to 0.2% rhenium.[16][31] Rhenium(VII) oxide and perrhenic acid readily dissolve in water; they are leached from flue dusts and gasses and extracted by precipitating with potassium or ammonium chloride as the perrhenate salts, and purified by recrystallization.[35] Total world production is between 40 and 50 tons/year; the main producers are in Chile, the United States, and Kazakhstan.[36] Recycling of used Pt-Re catalyst and special alloys allow the recovery of another 10 tons per year. Prices for the metal rose rapidly in early 2008, from $1000–$2000 per kg in 2003-2006 to over $10,000 in February 2008.[37][38] The metal form is prepared by reducing ammonium perrhenate with hydrogen at high temperatures:[35]
- 2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
Applications

Rhenium is added to high-temperature superalloys that are used to make jet engine parts, making 70% of the worldwide rhenium production.[39] Another major application is in platinum-rhenium catalysts, which are primarily used in making lead-free, high-octane gasoline.[36][40]
Alloys
The nickel-based superalloys have improved creep strength with the addition of rhenium. The alloys normally contain 3% or 6% of rhenium.[41] Second generation alloys contain 3%; these alloys were used in the engines of the F-16 and F-15, while the newer single-crystal third generation alloys contain 6% of rhenium; they are used in the F-22 and F-35 engines.[40][42] Rhenium is also used in the superalloys, such as CMSX-4 (2nd gen) and CMSX-10 (3rd gen) that are used in industrial gas turbine engines like the GE's 7FA. Rhenium can cause superalloys to become microstructurally unstable, forming undesirable TCP (topologically close packed) phases. In 4th and 5th generation superalloys, ruthenium is used to avoid this effect. Among others the new superalloys are EPM-102 (with 3 % Ru) and TMS-162 (with 6 % Ru), both contain 6 % rhenium,[43] as well as TMS-138[44] and TMS-174.[45][46]
For 2006, the consumption is given as 28% for General Electric, 28% Rolls-Royce plc and 12% Pratt & Whitney, all for superalloys, while the use for catalysts only accounts for 14% and the remaining applications use 18%.[39] In 2006, 77% of the rhenium consumption in the United States was in alloys.[40]
Rhenium improves the properties of tungsten and is therefore the most important alloying material for tungsten. Tungsten-rhenium alloys are more ductile at low temperature making them easier to machine, while the high-temperature stability is also improved. The effect increases with the rhenium concentration, and therefore tungsten alloys are produced with up to 27% of Re, which is the solubility limit.[47] One application for the tungsten-rhenium alloys is X-ray sources. The high melting point of both compounds, together with the high atomic mass, makes them stable against the prolonged electron impact.[48] Rhenium tungsten alloys are also applied as thermocouples to measure temperatures up to 2200 °C.[49]
The high temperature stability, low vapor pressure, good wear resistance and ability to withstand arc corrosion of rhenium are useful in self-cleaning electrical contacts. In particular, the discharge occurring during the switching oxidizes the contacts. However, rhenium oxide Re2O7 has poor stability (sublimates at ~360 °C) and therefore is removed during the discharge.[39]
Rhenium has a high melting point and a low vapor pressure similar to tantalum and tungsten, however, rhenium forms no volatile oxides. Therefore, rhenium filaments exhibit a higher stability if the filament is operated not in vacuum, but in oxygen-containing atmosphere.[50] Those filaments are widely used in mass spectrographs, in ion gauges.[51] and in photoflash lamps in photography.[52]
Catalysts
Rhenium in the form of rhenium-platinum alloy is used as catalyst for catalytic reforming, which is a chemical process to convert petroleum refinery naphthas with low octane ratings into high-octane liquid products. Worldwide, 30% of catalysts used for this process contain rhenium.[53] The olefin metathesis is the other reaction for which rhenium is used as catalyst. Normally Re2O7 on alumina is used for this process.[54] Rhenium catalysts are very resistant to chemical poisoning from nitrogen, sulfur and phosphorus, and so are used in certain kinds of hydrogenation reactions.[8][55][56]
Other uses
188Re and 186Re isotopes are radioactive and are used for treatment of liver cancer. They both have similar penetration depth in tissue (5 mm for 186Re and 11 mm for 188Re), but 186Re has advantage of longer lifetime (90 hours vs. 17 hours).[57][58]
Related by periodic trends, rhenium has a similar chemistry with technetium; work done to label rhenium onto target compounds can often be translated to technetium. This is useful for radiopharmacy, where it is difficult to work with technetium – especially the 99m isotope used in medicine – due to its expense and short half-life.[57][59]
Precaution
Very little is known about the toxicity of rhenium and its compounds because they are used in very small amounts. Soluble salts, such as the rhenium halides or perrhenates, could be hazardous due to elements other than rhenium or due to rhenium itself.[60] Only a few compounds of rhenium have been tested for their toxicity; two examples are potassium perrhenate and rhenium trichloride, which were injected as a solution into rats. The perrhenate had an LD50 value of 2800 mg/kg after seven days (this is very low, similar to that of table salt) and the rhenium trichloride showed LD50 of 280 mg/kg.[61]
References
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ Tilgner, Hans Georg (2000) (in German). Forschen Suche und Sucht. Books on Demand. ISBN 9783898112727. http://books.google.com/?id=UWBWnMOGtMQC.
- ↑ "Rhenium: Statistics and Information". Minerals Information. United States Geological Survey. 2008. http://minerals.usgs.gov/minerals/pubs/commodity/rhenium/. Retrieved 2008-02-03.
- ↑ Moseley, Henry (1914). "High Frequency Spectra of the Elements, Part II". Philosophical Magazine: 703–713. http://www.chemistry.co.nz/henry_moseley_article.htm.
- ↑ Noddack, W.; Tacke, I.; Berg, O. (1925). "Die Ekamangane". Naturwissenschaften 13 (26): 567–574. doi:10.1007/BF01558746.
- ↑ Noddack, W.; Noddack, I. (1929). "Die Herstellung von einem Gram Rhenium" (in German). Zeitschrift für anorganische und allgemeine Chemie 183 (1): 353–375. doi:10.1002/zaac.19291830126.
- ↑ Yoshihara, H. K. (2004). "Discovery of a new element ‘nipponiumʼ: re-evaluation of pioneering works of Masataka Ogawa and his son Eijiro Ogawa". Spectrochimica Acta Part B Atomic Spectroscopy 59: 1305–1310. doi:10.1016/j.sab.2003.12.027.
- ↑ 8.0 8.1 Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304857.
- ↑ Neshpor, V. S.; Novikov, V. I.; Noskin, V. A.; Shalyt, S. S. (1968). "Superconductivity of Some Alloys of the Tungsten-rhenium-carbon System". Soviet Physics JETP 27: 13. Bibcode: 1968JETP...27...13N.
- ↑ Daunt, J. G.; Smith, T. S. (1952). "Superconductivity of Rhenium". Physical Review 88 (2): 309–311. doi:10.1103/PhysRev.88.309.
- ↑ Daunt, J. G.; Lerner, E.. "The Properties of Superconducting Mo-Re Alloys". Defense Technical Information Center. http://stinet.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD0622881.
- ↑ Johnson, Bill (1993). "How to Change Nuclear Decay Rates". http://math.ucr.edu/home/baez/physics/ParticleAndNuclear/decay_rates.html. Retrieved 2009-02-21.
- ↑ Bosch; Faestermann, T; Friese, J; Heine, F; Kienle, P; Wefers, E; Zeitelhack, K; Beckert, K et al. (1996). "Observation of bound-state β– decay of fully ionized 187Re:187Re-187Os Cosmochronometry". Physical Review Letters 77 (26): 5190–5193. doi:10.1103/PhysRevLett.77.5190. PMID 10062738.
- ↑ Georges, Audi (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ 15.0 15.1 Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). "Rhenium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1118–1123. ISBN 3110075113.
- ↑ 16.0 16.1 16.2 16.3 16.4 Woolf, A. A. (1961). "An outline of rhenium chemistry". Quarterly Review of the Chemical Society 15: 372–391. doi:10.1039/QR9611500372.
- ↑ Schwochau, Klaus (2000). Technetium: chemistry and radiopharmaceutical applications. Wiley-VCH. p. 146. ISBN 3527294961. http://books.google.com/?id=BHjxH8q9iukC&pg=146.
- ↑ Goodman, J. T.; Rauchfuss, T. B. (2002). "Tetraethylammonium-tetrathioperrhenate [Et4N][ReS4]". Inorganic Syntheses 33: 107–110.
- ↑ Breimair, Josef; Steimann, Manfred; Wagner, Barbara; Beck, Wolfgang (1990). "Nucleophile Addition von Carbonylmetallaten an kationische Alkin-Komplexe [CpL2M(η2-RC≡CR)]+ (M = Ru, Fe): μ-η1:η1-Alkin-verbrückte Komplexe". Chemische Berichte 123: 7. doi:10.1002/cber.19901230103.
- ↑ Schmidt, Steven P.; Trogler, William C.; Basolo, Fred (1990). "Pentacarbonylrhenium Halides". Inorganic Syntheses 28: 154–159. doi:10.1002/9780470132593.ch42.
- ↑ Michael A. Urbancic, John R. Shapley (1990). "Pentacarbonylhydridorhenium". Inorganic Syntheses 28: 165–168. doi:10.1002/9780470132593.ch43.
- ↑ Lazarova, N.; James, S.; Babich, J.; Zubieta, J. (2004). "A convenient synthesis, chemical characterization and reactivity of [Re(CO)3(H2O)3]Br: the crystal and molecular structure of [Re(CO)3(CH3CN)2Br]". Inorganic Chemistry Communications 7 (9): 1023–1026. doi:10.1016/j.inoche.2004.07.006.
- ↑ Alberto, R.; Egli, A.; Abram, U.; Hegetschweiler, K.; Gramlich V.; Schubiger, P. A. (1994). "Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(µ3-OH)(µ-OH)3(CO)9] and [NEt4][Re2(µ-OH)3(CO)6] by alkaline titration". J. Chem. Soc., Dalton Trans.: 2815–2820. doi:10.1039/DT9940002815.
- ↑ Qin, Jiaqian; He, Duanwei; Wang, Jianghua; Fang, Leiming; Lei, Li; Li, Yongjun; Hu, Juan; Kou, Zili; Bi, Yan (2008). "Is Rhenium Diboride a Superhard Material?". Advanced Materials 20: 4780–4783. doi:10.1002/adma.200801471.
- ↑ doi:10.1021/j150552a005
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1021/j150511a004
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1016/0022-1902(60)80083-8
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Kenneth Malcolm Mackay; Rosemary Ann Mackay; W. Henderson (2002). Rosemary Ann Mackay. ed. Introduction to modern inorganic chemistry (6th ed.). CRC Press. pp. 368–369. ISBN 0748764208.
- ↑ Green, M. L. H.; Jones, D. J. (1965). Emeleus, H.J.; Sharpe, A.G.. ed. Advances in inorganic chemistry and radiochemistry. Academic Press. pp. 169–172. ISBN 0120236079.
- ↑ Emsley, John (2001). "Rhenium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 358–360. ISBN 0-19-850340-7. http://books.google.com/?id=j-Xu07p3cKwC.
- ↑ 31.0 31.1 Rouschias, George (1974). "Recent advances in the chemistry of rhenium". Chemical Reviews 74: 531. doi:10.1021/cr60291a002.
- ↑ Anderson, Steve T. "2005 Minerals Yearbook: Chile" (PDF). United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/country/2005/cimyb05.pdf. Retrieved 2008-10-26.
- ↑ Korzhinsky, M.A.; Tkachenko, S. I.; Shmulovich, K. I.; Taran Y. A.; Steinberg, G. S. (2004-05-05). "Discovery of a pure rhenium mineral at Kudriavy volcano". Nature 369: 51–52. doi:10.1038/369051a0.
- ↑ "The Mineral Rheniite". Amethyst Galleries. http://www.galleries.com/minerals/sulfides/rheniite/rheniite.htm.
- ↑ 35.0 35.1 Patnaik, Pradyot (2003). Handbook of Inorganic Chemicals. McGraw-Hill. pp. 790. ISBN 0070494398. OCLC 47726843.
- ↑ 36.0 36.1 Magyar, Michael J. (January 2008). "Rhenium" (PDF). Mineral Commodity Summaries. U.S. Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/rhenium/mcs-2008-rheni.pdf. Retrieved 2008-02-17.
- ↑ "MinorMetal prices". minormetals.com. http://www.minormetals.com/. Retrieved 2008-02-17.
- ↑ Harvey, Jan (2008-07-10). "Analysis: Super hot metal rhenium may reach "platinum prices"". Reuters India. http://in.reuters.com/article/oilRpt/idINL1037587920080710. Retrieved 2008-10-26.
- ↑ 39.0 39.1 39.2 Naumov, A. V. (2007). "Rhythms of rhenium". Russian Journal of Non-Ferrous Metals 48 (6): 418–423. doi:10.3103/S1067821207060089.
- ↑ 40.0 40.1 40.2 Magyar, Michael J.. "Mineral Yearbook: Rhenium" (PDF). United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/rhenium/myb1-2006-rheni.pdf.
- ↑ Bhadeshia, H. K. D. H.. "Nickel Based Superalloys". University of Cambridge. http://www.msm.cam.ac.uk/phase-trans/2003/Superalloys/superalloys.html. Retrieved 2008-10-17.
- ↑ Cantor, B.; Grant, Patrick Assender Hazel (2001). Aerospace Materials: An Oxford-Kobe Materials Text. CRC Press. pp. 82–83. ISBN 9780750307420. http://books.google.com/?id=n09-HajhRHYC.
- ↑ Bondarenko, Yu. A.; Kablov, E. N.; Surova, V. A.; Echin, A. B. (2006). "Effect of high-gradient directed crystallization on the structure and properties of rhenium-bearing single-crystal alloy". Metal Science and Heat Treatment 48: 360. doi:10.1007/s11041-006-0099-6.
- ↑ "Fourth generation nickel base single crystal superalloy". http://sakimori.nims.go.jp/catalog/TMS-138-A.pdf.
- ↑ Koizumi, Yutaka et al.. "Development of a Next-Generation Ni-base Single Crystal Superalloy". Proceedings of the International Gas Turbine Congress, Tokyo November 2–7, 2003. http://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_ts119.pdf.
- ↑ Walston, S.; Cetel, A.; MacKay, R.; O'Hara, K.; Duhl, D.; Dreshfield, R.. "Joint Development of a Fourth Generation Single Crystal Superalloy". http://gltrs.grc.nasa.gov/reports/2004/TM-2004-213062.pdf.
- ↑ Lassner, Erik; Schubert, Wolf-Dieter (1999). Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Springer. p. 256. ISBN 9780306450532. http://books.google.com/?id=foLRISkt9gcC&pg=PA256.
- ↑ Cherry, Pam; Duxbury, Angela (1998). Practical radiotherapy physics and equipment. Cambridge University Press. p. 55. ISBN 9781900151061. http://books.google.com/?id=5WIBbmmDm-gC&pg=PA55.
- ↑ Asamoto, R.; Novak, P. E. (1968). "Tungsten-Rhenium Thermocouples for Use at High Temperatures". Review of Scientific Instruments 39: 1233. doi:10.1063/1.1683642. http://link.aip.org/link/?RSINAK/39/1233/1.
- ↑ Blackburn, Paul E. (1966). "The Vapor Pressure of Rhenium". The Journal of Physical Chemistry 70: 311–312. doi:10.1021/j100873a513.
- ↑ Earle, G. D.; Medikonduri, R.; Rajagopal, N.; Narayanan, V.; Roddy, P. A. (2005). "Tungsten-Rhenium Filament Lifetime Variability in Low Pressure Oxygen Environments". IEEE Transactions on Plasma Science 33 (5): 1736–1737. doi:10.1109/TPS.2005.856413.
- ↑ Ede, Andrew (2006). The chemical element: a historical perspective. Greenwood Publishing Group. ISBN 9780313333040.
- ↑ Ryashentseva, Margarita A. (1998). "Rhenium-containing catalysts in reactions of organic compounds". Russian Chemical Reviews 67: 157–177. doi:10.1070/RC1998v067n02ABEH000390.
- ↑ Mol, Johannes C. (1999). "Olefin metathesis over supported rhenium oxide catalysts". Catalysis Today 51 (2): 289–299. doi:10.1016/S0920-5861(99)00051-6.
- ↑ Angelidis, T. N.; Rosopoulou, D. Tzitzios V. (1999). "Selective Rhenium Recovery from Spent Reforming Catalysts". Ind. Eng. Chem. Res. 38 (5): 1830–1836. doi:10.1021/ie9806242.
- ↑ Burch, Robert (1978). "The Oxidation State of Rhenium and Its Role in Platinum-Rhenium" (PDF). Platinum Metals Review 22 (2): 57–60. http://www.platinummetalsreview.com/pdf/pmr-v22-i2-057-060.pdf.
- ↑ 57.0 57.1 Dilworth, Jonathan R.; Parrott, Suzanne J. (1998). "The biomedical chemistry of technetium and rhenium". Chemical Society Reviews 27: 43–55. doi:10.1039/a827043z.
- ↑ "The Tungsten-188 and Rhenium-188 Generator Information". Oak Ridge National Laboratory. 2005. http://www.ornl.gov/sci/nuclear_science_technology/nu_med/188info.htm. Retrieved 2008-02-03.
- ↑ Colton, R.; Peacock R. D. (1962). "An outline of technetium chemistry". Quarterly Reviews Chemical Society 16: 299–315. doi:10.1039/QR9621600299.
- ↑ Emsley, J. (2003). "Rhenium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 358–361. ISBN 0198503407.
- ↑ Haley, Thomas J.; Cartwright, Frank D. (1968). "Pharmacology and toxicology of potassium perrhenate and rhenium trichloride". Journal of Pharmaceutical Sciences 57 (2): 321–323. doi:10.1002/jps.2600570218. PMID 5641681.
External links
- WebElements.com - Rhenium
- pure Rhenium >99,99% picture in the element collection from Heinrich Pniok
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||