Rutherfordium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unknown | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | rutherfordium, Rf, 104 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˌrʌðərˈfɔrdiəm/ RUDH-ər-FOR-dee-əm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 4, 7, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [261.11]g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d2 7s2 (predicted)[1] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 10, 2 (predicted) (Image) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 23 (estimated)[1] g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2400 (estimated)[1] K, 2100 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 5800 (estimated)[1] K, 5500 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +4 (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 490 (estimated)[1] kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 53850-36-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of rutherfordium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Rutherfordium (pronounced /ˌrʌðərˈfɔrdiəm/ RUDH-ər-FOR-dee-əm) is a chemical element with the symbol Rf and atomic number 104. On the periodic table of the elements, it is a p-block element and the first one of the transactinide element. It is member of the 7th period and also belongs to the group 4 elements. Chemistry experiments have confirmed that rutherfordium behaves as the heavier homologue to hafnium in group 4. Rutherfordium is a radioactive synthetic element whose most stable known isotope is 267Rf with a half-life of approximately 1.3 hours.
Small amounts of rutherfordium have been produced by bombarding plutonium-242 with accelerated neon-22 or californium-249 with accelerated carbon-12 ions in the 1960s. The priority of the discovery and therefore the naming of the element was disputed between Russian and American scientists and a final decision was taken in 1997 naming the element rutherfordium to honor New Zealand physicist Ernest Rutherford. Improved experimental techniques allowed to characterize some chemical properties of rutherfordium, which fit well into the chemistry of the other group 4 elements. Some calculations indicated that the element might show significantly different properties due to relativistic effects.
Contents |
History
Discovery
Element 104 was reportedly first detected in 1966 at the Joint Institute of Nuclear Research at Dubna (then in Soviet Union). Researchers there bombarded 242Pu with accelerated 22Ne ions and separated the reaction products by gradient thermochromatography after conversion to chlorides by interaction with ZrCl4. The team identified a spontaneous fission activity contained within a volatile chloride portraying eka-hafnium properties. Although a half-life was not accurately determined, later calculations indicated that the product was most likely 259Rf:[3]
- 24294Pu + 2210Ne → 264−x104Rf → 264−x104RfCl4
In 1969 researchers at the University of California, Berkeley conclusively synthesized the element by bombarding a californium-249 target with carbon-12 ions and measured the alpha decay of 257104, correlated with the daughter decay of 253102.[4]
- 24998Cf + 126C → 257104Rf + 4 n
The American synthesis was independently confirmed in 1973 and secured the identification of element 104 as the parent by the observation of K-alpha X-rays in the elemental signature of the daughter 253No. [5]
Naming controversy
The Russian scientists proposed the name kurchatovium and the American scientists suggested the name rutherfordium for the new element.[6] In 1992 the IUPAC/IUPAP Transfermium Working Group (TWG) assessed the claims of discovery and concluded that both teams provided contemporaneous evidence to the synthesis of element 104 and that credit should be shared between the two groups.[3]


The American group wrote a scathing response to the findings of the TWG, stating that they had given too much emphasis on the results from the Dubna group. In particular they pointed out that the Russian group had altered the details of their claims several times over a period of 20 years, a fact that the Russian team does not deny. They also stressed that the TWG had given too much credence to the chemistry experiments performed by the Russians and accused the TWG of not having appropriately qualified personnel on the committee. The TWG responded by saying that this was not the case and having assessed each point raised by the American group said that they found no reason to alter their conclusion regarding priority of discovery.[7] The IUPAC finally used the name suggested by the American team (rutherfordium) which may in some way reflect a change of opinion.[8]
As a consequence of the initial competing claims of discovery, an element naming controversy arose. Since the Soviets claimed to have first detected the new element they suggested the name kurchatovium, Ku, in honor of Igor Kurchatov (1903–1960), former head of Soviet nuclear research. This name had been used in books of the Soviet Bloc as the official name of the element. The Americans, however, proposed rutherfordium (Rf) for the new element to honor Ernest Rutherford, who is known as the "father" of nuclear physics. The International Union of Pure and Applied Chemistry (IUPAC) adopted unnilquadium, Unq, as a temporary, systematic element name, derived from the Latin names for digits 1, 0, and 4. In 1994, IUPAC suggested the name dubnium to be used since rutherfordium was suggested for element 106 and IUPAC felt that the Dubna team should be rightly recognized for their contributions. However, there was still a dispute over the names of elements 104−107. However, in 1997 the teams involved resolved the dispute and adopted the current name rutherfordium.[8]
The chemical properties of rutherfordium were based on calculation which indicated that the relativistic effects on the electron shell might be strong enough that the p orbitals have a lower energy level then the s orbitals and therefore the element more behaves like lead. With better calculation methods and studies of the chemical properties of rutherfordium compounds it could be shown that rutherfordium behaves according to the rest of the group 4 elements.[9]
Nucleosynthesis
Super heavy elements such as rutherfordium are produced by the bombardment of lighter elements using particle accelerators namely using fusion reactions to create them. Depending on the energies involved, these can be either cold fusion or hot fusion reactions. Whereas most of the isotopes of rutherfordium can be synthesized directly this way, some of the heavier ones have only been observed as decay products of elements with higher atomic numbers.
Hot fusion studies
In hot fusion reactions, very heavy targets (actinides) together with very light, high-energy projectiles are used. This gives rise to compound nuclei at high excitation energy (~40–50 MeV) that may either fission or evaporate several (3 to 5) neutrons. The synthesis of element 104 was first attempted in 1964 by the team at Dubna using the hot fusion reaction of neon projectiles with plutonium targets in the fusion reaction:
- 24294Pu + 2210Ne → 264-x104Rf + x n (x=3, 4?, or 5?)
The first study produced evidence for a spontaneous fission (SF) activity with a 0.3 second half-life, tentatively assigned to 260104 or 259104, and an unidentified SF activity at 8 seconds half-time. The former activity was later retracted and the latter activity associated with the now-known 259104 isotope.[3]
In 1966, in their discovery experiment, the team repeated the same reaction using a chemical study of volatile chloride products. The group identified a volatile chloride decaying by short spontaneous fission with eka-hafnium properties. This gave strong evidence for the formation of [104]Cl4 and the team suggested the name kurchatovium. Although a half-life was not accurately measured, later evidence suggested that the product was most likely 259104.[3] In 1968, the team searched for alpha decay from 260104 but were unable to detect such activity, but in 1970 repeated the chemistry experiment and obtained identical results to their 1966 experiment. In 1971, the reaction was repeated again and 0.1 s and 4.5 s SF activities were found. The 4.5 s activity was correctly assigned to 259104.[3] Later, the 0.1−0.3 s SF activity was retracted as belonging to a kurchatovium isotope but the observation of eka-hafnium reactivity remained and was the basis of their successful claim to discovery.[3] The reaction was further studied in 2000 by Yuri Lazarev at Dubna who observed 261Rf, later reassigned to 261mRf. A similar reaction was studied in 1964 by the researchers at Dubna to assist in the original assignments, using a different neon-20 isotope, but were unable to detect any 0.3 s spontaneous fission activities.[3] Later studies in 2003 at the Paul Scherrer Institute (PSI) in Bern, detected some SF activities but were unable to confirm the formation of 259Rf.[10]
Researchers at the University of California led by Albert Ghiorso tried to detect in 1969 the 0.1–0.3 s SF activity reported at Dubna, assigned to 260104, from a curium and oxygen reaction:
- 24896Cm + 168O → 264-x104Rf + x n (x=4).
They were unable to do so, only observing a 10–30 ms SF activity, correctly assigned to 260104. The failure to observe the 0.3 s SF activity identified by Dubna gave the Americans the incentive to name this element rutherfordium. Later experiments in 1976 with curium-246, lead the Americans to associate the Soviet's original observations to background signals.[3] In 1969, the Californian team also investigated the reaction of californium with carbon and observed an 11 ms SF activity which they correctly assigned to 258104:[4]
- 24998Cf + 136C → 262-x104Rf + x n (x=4).
In their 1969 discovery experiments, the team at University of California also used carbon-12 beam instead of carbon-13 to irradiate a californium-249 target, and they confirmed the 11 ms SF activity assigned to 258104. However, the actual discovery experiment was the observation of alpha decays linked to 253102, and therefore positively identified as 257104.[4] In 1973, Bemis and his team at Oak Ridge confirmed the discovery by measuring coincident X-rays from the daughter 253102.[5]
In 1970 Albert Ghiorso's team also studied the reaction of curium with oxygen-18 and identified 261Rf with a half life of 65 seconds.[11] In a 1981 study at LBNL, a 1.5 s SF activity was identified and assigned to a fermium descendant, but later evidence indicates a possible assignment to 262Rf. In contrast, in a subsequent review of isotope properties by Somerville et al. at LBNL in 1985, a 47 ms SF activity was assigned to 262Rf, but this has not been verified.[12] Further studies in 1991 at the LBNL indicated spontaneous fission activities with long lifetimes, tentatively assigned to 263Rf. In 1996, chemical studies on rutherfordium chloride were published by the LBNL, in which the half-life was improved to 78 s. A repeat of the experiment in 2000 assessing the volatility of the bromide further refined the half-life to 75 s.
The reaction of berkelium-249 with nitrogen-15 was studied first in 1977 in Dubna, and eventually they confirmed in 1985 the formation of the 260104 isotope with a SF activity of 28 ms:[3]
- 24997Bk + 147N → 264-x104Rf + x n (x=4).
Heavier isotopes were synthesized only much later. In 1996, the isotope 262Rf was first observed in LBNL from the reaction:
- 24494Pu + 2210Ne → 266-x104Rf + x n (x=4, 5).
The team detected the spontaneous fission (SF) of 262Rf and determine its half-life as 2.1 s, in contrast to earlier reports of a 47 ms activity. It was suggested that the two half-lives might be related to different isomeric states.[13] The reaction was further studied in 2000 by Yuri Lazarev and the team at Dubna which observed 69 alpha decays from 261Rf and spontaneous fission of 262Rf.[14] Later work on hassium has allowed a reassignment of the former product to 261mRf.
The hot fusion reaction using a uranium target was first reported in 2000 by Yuri Lazarev and the team at the Flerov Laboratory of Nuclear Reactions (FLNR):
- 23892U + 2612Mg → 264-x104Rf + x n (x=3, 4, 5, 6).
They observed decays from 260Rf and 259Rf.[15] In 2006, as part of their program on the study of uranium targets in hot fusion reactions, the team at LBNL observed also 261Rf.[16][17]
Cold fusion studies
In cold fusion reactions, the produced fused nuclei have a low excitation energy (~10-20 MeV) that increased the probability of these products to survive fission. As the fused nuclei cool to the ground state, they only require the emission of only one or two neutrons.[18] The attempted reactions used light titanium nuclei aimed at targets consisting of lead isotopes. The first such reaction was studied in 1974 by the team at Dubna:
- 20882Pb + 5022Ti → 258-x104Rf + x n (x=1, 2, or 3)
The measurement of a spontaneous fission activity was assigned to 256Rf.[19] Later, in 1985, this reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotopes 257Rf and 256Rf.[20] After an upgrade of the facilities, the team as GSI repeated the reaction in 1994 with much higher sensitivity and detected some 1100 atoms of 257Rf and 1900 atoms of 256Rf along with 255Rf, and isomeric levels for 257Rf and 255Rf.[21] The reaction was further studied in 2002 by scientists at the Argonne University in Illinois,[22] while in 2007, the Lawrence Berkeley National Laboratory (LBNL) reported further isomers of 256Rf.[23]
Researchers at Dubna also investigated the reaction of 207Pb with 50Ti to produce 255Rf in 1974. The reaction was further studied in 1985 by the GSI team who measured the decay properties of the isotope 255Rf, and in 2000 they published the first decay level scheme for the isotope.[24] In a 1994 study by GSI with the 206Pb isotope instead, 255Rf and 144 atoms of the new isotope 254Rf were detected. The latter decayed by spontaneous fission.[21] 253Rf was similarly detected that year when 204Pb was used instead, and similarly decayed by spontaneous fission.[21]
Decay studies
No isotopes with an atomic mass of 262 have been synthesized by direct fusion reactions. Decay reactions of elements with higher atomic number have generated many of these isotopes, together with some heavier ones.
Investigations on the synthesis of the isotope 263Rf were done in 1999 at the University of Bern, Switzerland on the reaction:
A rutherfordium fraction was separated and several SF events with long lifetimes and alpha decays with energy 7.8 MeV and 7.9 MeV were observed. A second experiment using a study of the fluoride of rutherfordium products also produced 7.9 MeV alpha decays.[25]
Isotopes of rutherfordium have also been identified in the decay of heavier elements. Observations to date are summarized in the table below. EC refers to electron capture.
| Evaporation residue | Observed Rf isotope |
|---|---|
| 288Uup | 268Rf (possible EC of 268Db) |
| 291Uuh, 287Uuq, 283Cn | 267Rf |
| 282Uut | 266Rf (EC of 266Db) |
| 271Hs | 263gRf |
| 263Db | 263mRf (EC of 263Db) |
| 266Sg (possibly 266mSg) | 262Rf (possibly 262mRf) |
| 277Cn, 273Ds, 269Hs, 265Sg | 261mRf, 261Rf |
| 271Ds, 267Hs, 263Sg | 259Rf |
| 269Ds, 265Hs, 261Sg | 257Rf |
| 264Hs, 260Sg | 256Rf |
| 259Sg | 255Rf |
Isotopes
| Isotope |
Discovery year |
Reaction |
|---|---|---|
| 253Rf | 1994 | 204Pb(50Ti,n)[21] |
| 254Rf | 1994 | 206Pb(50Ti,2n)[21] |
| 255Rf | 1974 | 207Pb(50Ti,2n)[24] |
| 256Rf | 1974 | 208Pb(50Ti,2n)[24] |
| 257Rf | 1969 | 249Cf(12C,4n)[4] |
| 258Rf | 1969 | 249Cf(13C,4n)[4] |
| 259Rf | 1969 | 249Cf(13C,3n)[4] |
| 260Rf | 1969 | 248Cm(16O,4n)[3] |
| 261Rf | 1970 | 248Cm(18O,5n)[11] |
| 262Rf | 1996 | 244Pu(22Ne,4n) [13] |
| 263Rf | 1999 | 248Cm(22Ne,α,3n)[25] |
| 264Rf | unknown | - |
| 265Rf | unknown | - |
| 266Rf? | 2006 | 237Np(48Ca,3n) [note 1] |
| 267Rf | 2003/2004 | 238U(48Ca,3n) [note 2] |
| 268Rf? | 2003 | 243Am(48Ca,3n) [note 3] |
Rutherfordium does not have any stable or naturally occurring isotopes. However, a several radioactive isotopes have been synthesized in the laboratory, either by fusing two atoms (see next section), or by observing the decay of heavier elements that rutherfordium. At least 15 different isotopes have been reported so far ranging in their atomic mass from 253 up to 268.[2]
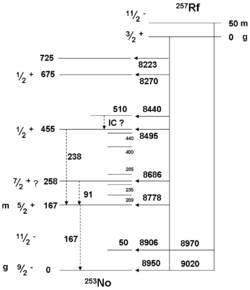
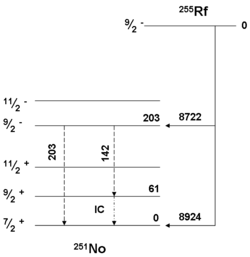
The lighter isotopes usually have shorter half lives, e.g., under 50 μs for 253Rf and 254Rf. 256Rf, 258Rf, 260Rf are more stable at around 10 ms, 255Rf, 257Rf, 259Rf, and 262Rf live between 1 and 5 seconds, and 261Rf and 263Rf are more stable, at around 1 and 10 min, respectively. The heaviest known isotopes are the most stable, with 265Rf having a reported half-life of about 13 h. Isotopes 264Rf, 266Rf and 268Rf have also been observed to have long half-lives of 1 h, 10 h and 6 h, respectively, but these have only been measured indirectly through systematic studies.[2]
Other
The lightest isotopes were synthesized by direct fusion between two lighter nuclei. The heaviest isotope produced this way is 262Rf, meaning that the remaining ones have only been observed as decay products of elements with larger atomic numbers.
Unconfirmed and retracted isotopes
- 268Rf
In the synthesis of ununpentium, the isotope 288115 decayed to 268Db which undergoes spontaneous fission with a half-life of 29 hours. Given that the electron capture of 268Db cannot be detected, these SF events may be due to the SF of 268Rf, in which case the half-life of this isotope cannot be extracted.[note 3]
- 266Rf
In the synthesis of ununtrium, the isotope 282113 decayed to 266Db which undergoes spontaneous fission with a half-life of 22 minutes. Given that the electron capture of 266Db cannot be detected, these SF events may be due to the SF of 266Rf, in which case the half-life of this isotope cannot be extracted. [note 1]
- 265Rf
In 1999, American scientists at the University of California, Berkeley, announced that they had succeeded in synthesizing three atoms of 293118. These parent nuclei successively emitted seven alpha particles to form 265Rf nuclei. Their claim was retracted in 2001. As such, this rutherfordium isotope is unconfirmed or unknown.[note 4]
Nuclear isomerism
Initial work on the synthesis of rutherfordium isotopes by hot fusion pathways focused on the synthesis of 263Rf. Several studies have indicated that this nuclide decays primarily by spontaneous fission with a long half-life of 10–20 minutes. Alpha particles with energy of 7.8−7.9 MeV have also been associated with this nucleus. More recently, a study of hassium isotopes allowed the synthesis of an atom of 263Rf decaying by spontaneous fission with a short half-life of 8 seconds. These two different decay modes must be associated with two isomeric states. Specific assignments are difficult due to the low number of observed events. Further studies are required to allow definite assignments.[27]
Early research on the synthesis of rutherfordium isotopes utilized the 244Pu(22Ne,5n)261Rf reaction. The product was found to undergo exclusive 8.28 MeV alpha decay with a half-life of 78 seconds. Later studies by the GSI team on the synthesis of copernicium and hassium isotopes produced conflicting data. In this case, 261Rf was found to undergo 8.52 MeV alpha decay with a short half-life of 4 seconds. Later results indicated a predominant fission branch. These contradictions led to some doubt on the discovery of copernicium. The first isomer is currently denoted 261aRf whilst the second is denoted 261bRf. However, it is thought that the first nucleus belongs to a high-spin ground state and the latter to a low-spin metastable state. [28] The discovery and confirmation of 261bRf provided proof for the discovery of copernicium in 1996.
A detailed spectroscopic study of the production of 257Rf nuclei using the reaction 208Pb(50Ti,n)257Rf allowed the identification of an isomeric level in 257Rf. The work confirmed that 257gRf has a complex spectrum with 15 alpha lines. A level structure diagram was calculated for both isomers.[29]
Chemical properties
Group 4 membership
Element 104 is the first member of the 6d series of transition metals and the heaviest member of group IV in the periodic table, below titanium, zirconium and hafnium. Some of its properties were determined by gas-phase experiments and aqueous chemistry. The IV oxidation state is the only stable state for the latter two elements and therefore rutherfordium should also portray a stable +4 state.[9]
In an analogous manner to zirconium and hafnium, rutherfordium is projected to form a very stable, high melting point oxide, RfO2. It reacts with halogens to form tetrahalides, RfX4, which hydrolyze on contact with water to form oxyhalides RfOX2. The tetrahalides are volatile solids existing as monomeric tetrahedral molecules in the vapor phase.[9]
In the aqueous phase, the Rf4+ ion hydrolyzes less than titanium(IV) and to a similar extent to zirconium and hafnium, thus leading to the rutherfordyl oxyion, RfO2+. Treatment of the halides with halide ions promotes the formation of complex ions. The use of chloride and bromide ion form the hexahalide complexes RfCl62− and RfBr62−. For the fluoride complexes, zirconium and hafnium tend to form hepta- and octa- complexes. Thus, for the larger rutherfordium ion, the complexes RfF62−, RfF73− and RfF84− are possible.[9]
Experimental chemistry
Gas phase
| Formula | Names |
|---|---|
| RfCl4 | rutherfordium tetrachloride, rutherfordium(IV) chloride |
| RfBr4 | rutherfordium tetrabromide, rutherfordium(IV) bromide |
| RfOCl2 | rutherfordium oxychloride, rutherfordyl(IV) chloride, rutherfordium(IV) dichloride oxide |
| [RfCl6]2− | hexachlororutherfordate(IV) |
| [RfF6]2− | hexafluororutherfordate(IV) |
| K2[RfCl6] | potassium hexachlororutherfordate(IV) |
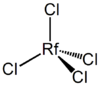
Early work on the study of the chemistry of rutherfordium focused on gas thermochromatography and measurement of relative deposition temperature adsorption curves. The initial work was carried out at Dubna in an attempt to reaffirm their discovery of the element. Recent work is more reliable regarding the identification of the parent rutherfordium radioisotopes. The isotope 261mRf has been used for these studies. The experiments relied on the expectation that rutherfordium would begin the new 6d series of elements and should therefore form a volatile tetrachloride due to the tetrahedral nature of the molecule.[9][30]
As series of experiments have confirmed that rutherfordium behaves as a typical member of group 4 forming a tetravalent chloride (RfCl4) and bromide (RfBr4) as well as an oxychloride (RfOCl2).[9][31]
Aqueous phase
Rutherfordium is expected to have the electron configuration [Rn]5f14 6d2 7s2 and therefore behave as the heavier homologue of hafnium in group 4 of the periodic table. It should therefore readily form a hydrated Rf4+ ion in strong acid solution and should readily form complexes in hydrochloric acid, hydrobromic or hydrofluoric acid solutions.[9]
The most conclusive aqueous chemistry studies of rutherfordium have been performed by the Japanese team at JAERI using the radioisotope 261mRf. Extraction experiments from hydrochloric acid solutions using isotopes of rutherfordium, hafnium, zirconium and thorium have proved a non-actinide behavior. A comparison with its lighter homologues placed rutherfordium firmly in group 4 and indicated the formation of a hexachlororutherfordate complex in chloride solutions, in a manner similar to hafnium and zirconium. [9] [32]
- 261mRf4+ + 6 Cl− → [261mRfCl6]2−
Very similar results were observed in hydrofluoric acid solutions. Differences in the extraction curves were interpreted as a weaker affinity for fluoride ion and the formation of the hexafluororutherfordate ion, whereas hafnium and zirconium ions complex seven or eight fluoride ions at the concentrations used:[9]
- 261mRf4+ + 6 F− → [261mRfF6]2−
Notes
- ↑ 1.0 1.1 see ununtrium
- ↑ see copernicium
- ↑ 3.0 3.1 see ununpentium
- ↑ see ununoctium
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Chemical Data. Rutherfordium - Rf, Royal Chemical Society
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. http://www.nndc.bnl.gov/chart/reCenter.jsp?z=104&n=158. Retrieved 2008-06-06.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Barber, R. C.; Greenwood, N. N.; Hrynkiewicz, A. Z.; Jeannin, Y. P.; Lefort, M.; Sakai, M.; Ulehla, I.; Wapstra, A. P.; Wilkinson, D. H. (1993). "Discovery of the transfermium elements. Part II: Introduction to discovery profiles. Part III: Discovery profiles of the transfermium elements". Pure and Applied Chemistry 65 (8): 1757–1814. doi:10.1351/pac199365081757.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Ghiorso, A.; Nurmia, M.; Harris, J.; Eskola, K.; Eskola, P. (1969). "Positive Identification of Two Alpha-Particle-Emitting Isotopes of Element 104". Physical Review Letters 22: 1317–1320. doi:10.1103/PhysRevLett.22.1317.
- ↑ 5.0 5.1 Bemis, C. E.; Silva, R.; Hensley, D.; Keller, O.; Tarrant, J.; Hunt, L.; Dittner, P.; Hahn, R. et al. (1973). "X-Ray Identification of Element 104". Physical Review Letters 31: 647–650. doi:10.1103/PhysRevLett.31.647.
- ↑ http://www.rsc.org/chemistryworld/podcast/Interactive_Periodic_Table_Transcripts/Rutherfordium.asp
- ↑ Ghiorso, A.; Seaborg, G. T.; Organessian, Yu. Ts.; Zvara, I.; Armbruster, P.; Hessberger, F. P.; Hofmann, S.; Leino, M.; Munzenberg, G.; Reisdorf W.; Schmidt, K.-H. (1993). "Responses on 'Discovery of the transfermium elements' by Lawrence Berkeley Laboratory, California; Joint Institute for Nuclear Research, Dubna; and Gesellschaft fur Schwerionenforschung, Darmstadt followed by reply to responses by the Transfermium Working Group". Pure and Applied Chemistry 65 (8): 1815–1824. doi:10.1351/pac199365081815.
- ↑ 8.0 8.1 "Names and symbols of transfermium elements (IUPAC Recommendations 1997)". Pure and Applied Chemistry 69 (12): 2471–2474. 1997. doi:10.1351/pac199769122471.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 Kratz, J. V. (2003). "Critical evaluation of the chemical properties of the transactinide elements (IUPAC Technical Report)". Pure and Applied Chemistry 75: 103. doi:10.1351/pac200375010103. http://stage.iupac.org/originalWeb/publications/pac/2003/pdf/7501x0103.pdf.
- ↑ "20Ne On 244Pu - First Preliminary Results". http://lch.web.psi.ch/files/anrep03/03.pdf.
- ↑ 11.0 11.1 Ghiorso, A.; Nurmia, M.; Eskola, K.; Eskola P. (1970). "261Rf; new isotope of element 104". Physics Letters B 32 (2): 95–98. doi:10.1016/0370-2693(70)90595-2.
- ↑ Somerville, L. P.; M. J. Nurmia, J. M. Nitschke, and A. Ghiorso E. K. Hulet and R. W. Lougheed (1985). "Spontaneous fission of rutherfordium isotopes". Physical Review C 31 (5): 1801–1815. doi:10.1103/PhysRevC.31.1801.
- ↑ 13.0 13.1 Lane, M. R.; Gregorich, K.; Lee, D.; Mohar, M.; Hsu, M.; Kacher, C.; Kadkhodayan, B.; Neu, M. et al. (1996). "Spontaneous fission properties of 104262Rf". Physical Review C 53 (6): 2893–2899. doi:10.1103/PhysRevC.53.2893.
- ↑ Lazarev, Yu. A.; Lobanov, Yu.; Oganessian, Yu.; Utyonkov, V.; Abdullin, F.; Polyakov, A.; Rigol, J.; Shirokovsky, I. et al. (1996). "Decay properties of 257No, 261Rf, and262Rf". Physical Review C 62 (6): 064307. doi:10.1103/PhysRevC.62.064307.
- ↑ Lazarev, Yu. A.; Lobanov, Yu. V.; Oganessian, Yu. Ts.; Utyonkov, V. K.; Abdullin, F. Sh.; Polyakov, A. N.; Rigol, J.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Iliev, S.; Subbotin, V. G.; Sukhov, A. M.; Buklanov, G. V.; Mezentsev, A. N.;Subotic, K.; Moody, K. J.; Stoyer, N. J.; Wild, J. F.; Lougheed, R. W. (2000). "Decay properties of 257No, 261Rf, and 262Rf". Physical Review C 62 (6): 064307. doi:10.1103/PhysRevC.62.064307.
- ↑ Gregorich, K.E.; Ch.E. Düllmann, C.M. Folden III, R. Sudowe, S.L. Nelson, J.M. Gates, I. Dragojević, M.A. Garcia, Y.H. Chung, R. Eichler, G.K. Pang, A. Türler, A. Yakushev, D.C. Hoffman, H. Nitsche (2005). "Systematic Study of Heavy Element Production in Compound Nucleus Reactions with 238U Targets". LBNL annual report. http://rnc.lbl.gov/nsd/annualreport2005/contributions/Gregorich_LE.pdf. Retrieved 2008-02-29.
- ↑ Gates, J. M.; Garcia, M.; Gregorich, K.; Düllmann, Ch.; Dragojević, I.; Dvorak, J.; Eichler, R.; Folden, C. et al. (2008). "Synthesis of rutherfordium isotopes in the 238U(26Mg,xn)264−xRf reaction and study of their decay properties". Physical Review C 77: 034603. doi:10.1103/PhysRevC.77.034603.
- ↑ Armbruster, Peter; Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American 34: 36–42.
- ↑ Oganessian, Yu.Ts.; Demin, A. G.; Il'inov, A. S.; Tret'yakova, S. P.; Pleve, A. A.; Penionzhkevich, Yu. É.; Ivanov M. P.; Tret'yakov, Yu. P. (1975). "Experiments on the synthesis of neutron-deficient kurchatovium isotopes in reactions induced by 50Ti Ions". Nuclear Physics A 38 (6): 492–501. doi:10.1016/0375-9474(75)91140-9.
- ↑ Heßberger, F. P.; Münzenberg, G.; Hofmann, S.; Reisdorf, W.; Schmidt, K. H.; Schött, H. J.; Armbruster, P.; Hingmann, R.; Thuma, B.; Vermeulen, D. (1985). "Study of evaporation residues produced in reactions of 207,208Pb with 50Ti". Zeitschrift für Physik a Atoms and Nuclei 321: 317. doi:10.1007/BF01493453.
- ↑ 21.0 21.1 21.2 21.3 21.4 Heßberger, F. P.; Hofmann, S.; Ninov, V.; Armbruster, P.; Folger, H.; Münzenberg, G.; Schött, H. J.; Popeko, A. K.; Yeremin, A. V. ; Andreyev, A. N.; Saro, S. (1997). "Spontaneous fission and alpha-decay properties of neutron deficient isotopes 257−253104 and 258106". Zeitschrift für Physik a Hadrons and Nuclei 359: 415. doi:10.1007/s002180050422.
- ↑ Qian, J.; Heinz, A.; Winkler, R.; Vinson, J.; Janssens, R. V. F.; Peterson, D.; Seweryniak, D.; Back, B.; Carpenter, M. P.; Savard, G.; Hecht, A. A.; Jiang, C. L.; Khoo, T. L.; Kondev, F. G.; Lauritsen, T.; Lister, C. J.; Robinson, A.; Wang, X.; Zhu, S.; Gansworthy, A. B.; Asai, M.. "Alpha decay of 257Rf". American Physical Society, 2007 Annual Meeting of the Division of Nuclear Physics: JG.010. http://adsabs.harvard.edu/abs/2007APS..DNP.JG010Q.
- ↑ Jeppesen, H.B.; Dragojević, I.; Clark, R.; Gregorich, K.; Ali, M.; Allmond, J.; Beausang, C.; Bleuel, D. et al. (2009). "Multi-quasiparticle states in 256Rf". Physical Review C 79: 031303(R). doi:10.1103/PhysRevC.79.031303.
- ↑ 24.0 24.1 24.2 Heßberger, F.P.; Hofmann, S.; Ackermann, D.; Ninov, V.; Leino, M.; Münzenberg, G.; Saro, S.; Lavrentev, A.; Popeko, A.G.; Yeremin, A.V.; Stodel, Ch. (2001). "Decay properties of neutron-deficient isotopes 256,257Db, 255Rf, 252,253Lr""]. European Physical Journal A 12: 57–67. doi:10.1007/s100500170039. http://www.edpsciences.org/articles/epja/abs/2001/09/epja1103/epja1103.html.
- ↑ 25.0 25.1 "An EC-branch in the decay of 27-s263Db: Evidence for the new isotope 263Rf", Kratz et al., GSI Annual report 2001. Retrieved on 2008-02-29
- ↑ Heßberger, F. P.; Hofmann, S.; Ackermann, D.; Antalic, S.; Kindler, B.; Kojouharov, I.; Kuusiniemi, P.; Leino, M. et al. (2006). "Alpha-gamma decay studies of 255Rf, 251No and 247Fm". The European Physical Journal A 30: 561. doi:10.1140/epja/i2006-10137-2.
- ↑ Kratz, J. V.; Nahler, A.; Rieth, U.; Kronenberg, A.; Kuczewski, B.; Strub, E.; Bruchle, W.; Schadel, M. et al. (2003). "An EC-branch in the decay of 27-s 263Db: Evidence for the isotope 263Rf". Radiochimica Acta 91: 59. doi:10.1524/ract.91.1.59.19010. ISBN 0874887992.
- ↑ "Evidence for isomeric states in 261Rf", Dressler et al., PSI Annual Report 2001. Retrieved on 2008-01-29
- ↑ Qian, J.; Heinz, A.; Khoo, T.; Janssens, R.; Peterson, D.; Seweryniak, D.; Ahmad, I.; Asai, M. et al. (2009). "Spectroscopy of Rf257". Physical Review C 79: 064319. doi:10.1103/PhysRevC.79.064319.
- ↑ Türler, A (1998). "Evidence for relativistic effects in the chemistry of element 104". Journal of Alloys and Compounds 271-273: 287. doi:10.1016/S0925-8388(98)00072-3.
- ↑ Gäggeler, Heinz W. (2007-11-05). "Lecture Course Texas A&M: Gas Phase Chemistry of Superheavy Elements". http://lch.web.psi.ch/files/lectures/TexasA&M/TexasA&M.pdf. Retrieved 2010-03-30.
- ↑ Nagame, Y.; Tsukada, K.; Asai, M.; Toyoshima, A.; Akiyama, K.; Ishii, Y.; Kaneko-Sato, T.; Hirata, M. et al. (2005). "Chemical studies on rutherfordium (Rf) at JAERI". Radiochimica Acta 93: 519. doi:10.1524/ract.2005.93.9-10.519. http://wwwsoc.nii.ac.jp/jnrs/paper/JN62/jn6202.pdf.
External links
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||