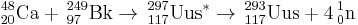Ununseptium
|
|||||||||||||||||||
| Appearance | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unknown | |||||||||||||||||||
| General properties | |||||||||||||||||||
| Name, symbol, number | ununseptium, Uus, 117 | ||||||||||||||||||
| Pronunciation | oon-oon-SEP-tee-əm |
||||||||||||||||||
| Category notes | Unknown | ||||||||||||||||||
| Group, period, block | 17, 7, p | ||||||||||||||||||
| Standard atomic weight | [294]g·mol−1 | ||||||||||||||||||
| Electron configuration | Unknown | ||||||||||||||||||
| Electrons per shell | 2,8,18,32,32,18,7 (predicted) (Image) |
||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| Miscellanea | |||||||||||||||||||
| CAS registry number | 54101-14-3 | ||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||
| Main article: Isotopes of ununseptium | |||||||||||||||||||
|
|||||||||||||||||||
Ununseptium (pronounced /uːnuːnˈsɛptiəm/ (![]() listen)[1] oon-oon-SEP-tee-əm) is the temporary name of the chemical element with temporary symbol Uus and atomic number 117. It is the latest element to have a claim of discovery, with six atoms having been detected by a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, in 2009–10.[2][3] Although it is placed as the heaviest member of the halogen family, no experimental data are available to support properties similar to the lighter members like astatine or iodine.
listen)[1] oon-oon-SEP-tee-əm) is the temporary name of the chemical element with temporary symbol Uus and atomic number 117. It is the latest element to have a claim of discovery, with six atoms having been detected by a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, in 2009–10.[2][3] Although it is placed as the heaviest member of the halogen family, no experimental data are available to support properties similar to the lighter members like astatine or iodine.
Contents |
History
Discovery
In January 2010, scientists at the Flerov Laboratory of Nuclear Reactions announced internally[3] that they had succeeded in detecting the decay of a new element with Z=117 using the reactions:
Just six atoms were synthesized of two neighbouring isotopes, neither of which decayed to known isotopes of lighter elements. Their results were published on 9 April 2010 in the journal Physical Review Letters.[4]
Naming
The element with atomic number 117 is historically known as eka-astatine (see 'eka' terminology). The name ununseptium is a systematic element name, used as a placeholder until the discovery is acknowledged by the IUPAC, and the IUPAC decides on a name. Usually, the name suggested by the discoverer(s) is chosen.
According to current guidelines from IUPAC, the ultimate name for all new elements should end in "-ium", which means the name for ununseptium may end in -ium, not -ine, even if ununseptium turns out to be a halogen.[5]
Current experiments
Scientists at Dubna are continuing their study of the 249Bk + 48Ca reaction in order to attempt a first chemical study of ununtrium.
Future experiments
The team at the GSI in Darmstadt, recently acknowledged as the discoverers of copernicium have begun experiments aiming towards a synthesis of ununseptium. The GSI have indicated that if they are unable to acquire any 249Bk from the United States, which is likely given the situation regarding the attempt in Russia, they will study the reaction 244Pu(51V,xn) instead, or possibly 243Am(50Ti,xn).[6]
Isotopes and nuclear properties
Nucleosynthesis
Target-projectile combinations leading to Z=117 compound nuclei
The below table contains various combinations of targets and projectiles which could be used to form compound nuclei with atomic number 117.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 81Br | 289Uus | Reaction yet to be attempted |
| 232Th | 59Co | 291Uus | Reaction yet to be attempted |
| 238U | 55Mn | 293Uus | Reaction yet to be attempted |
| 237Np | 54Cr | 291Uus | Reaction yet to be attempted |
| 244Pu | 51V | 295Uus | Reaction yet to be attempted |
| 243Am | 50Ti | 293Uus | Reaction yet to be attempted |
| 248Cm | 45Sc | 293Uus | Reaction yet to be attempted |
| 249Bk | 48Ca | 297Uus | Successful reaction |
| 249Cf | 41K | 290Uus | Reaction yet to be attempted |
Hot fusion
249Bk (48Ca, xn)297-xUus (x=3,4)
Between July 2009 and February 2010, the team at the JINR (Flerov Laboratory of Nuclear Reactions) ran a 7-month-long experiment to synthesize ununseptium using the reaction above.[7] The expected cross-section was of the order of 2 pb. The expected evaporation residues, 293Uus and 294Uus, were predicted to decay via relatively long decay chains as far as isotopes of dubnium or lawrencium.
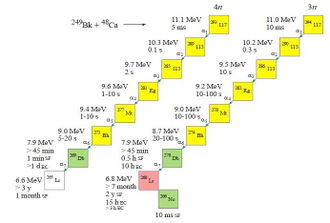 Calculated decay chains from the parent nuclei 293Uus and 294 Uus[8] |
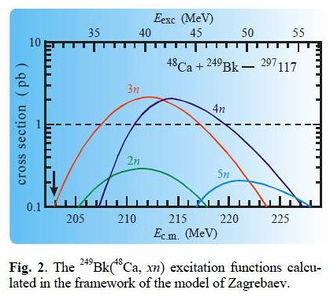 Calculated excitation function for the production of the compound nucleus 297Uus from the reaction 249Bk( 48Ca,xn) [8] |
The team published a scientific paper in April 2010 (first results were presented in January 2010[3]) that six atoms of the neighbouring isotopes 294Uus (one atom) and 293Uus (five atoms) were detected. The heavier isotope decayed by the successive emission of six alpha particles down as far as the new isotope 270Db which underwent apparent spontaneous fission. On the other hand, the lighter odd-even isotope decayed by the emission of just three alpha particles, as far 281Rg, which underwent spontaneous fission. The reaction was run at two different excitation energies of 35 MeV (dose 2x1019) and 39 MeV (dose 2.4×1019). Initial decay data was published as an preliminary presentation on the JINR website.[9]
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 294Uus | 2009 | 249Bk(48Ca,3n) |
| 293Uus | 2009 | 249Bk(48Ca,4n) |
Theoretical calculations
Evaporation residue cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
DNS = Di-nuclear system; σ = cross section
| Target | Projectile | CN | Channel (product) | σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 209Bi | 82Se | 291Uus | 1n (290Uus) | 15 fb | DNS | [10] |
| 209Bi | 79Se | 288Uus | 1n (287Uus) | 0.2 pb | DNS | [10] |
| 232Th | 59Co | 291Uus | 2n (289Uus) | 0.1 pb | DNS | [10] |
| 238U | 55Mn | 293Uus | 2-3n (291,290Uus) | 70 fb | DNS | [10] |
| 244Pu | 51V | 295Uus | 3n (292Uus) | 0.6 pb | DNS | [10] |
| 248Cm | 45Sc | 293Uus | 4n (289Uus) | 2.9 pb | DNS | [10] |
| 246Cm | 45Sc | 291Uus | 4n (287Uus) | 1 pb | DNS | [10] |
| 249Bk | 48Ca | 297Uus | 3n (294Uus) | 2.1 pb ; 3 pb | DNS | [10][11] |
| 247Bk | 48Ca | 295Uus | 3n (292Uus) | 0.8, 0.9 pb | DNS | [11][10] |
Decay characteristics
Theoretical calculations in a quantum tunneling model with mass estimates from a macroscopic-microscopic model predict the alpha-decay half-lives of isotopes of ununseptium (namely, 289–303117) to be around 0.1–40 ms.[12][13][14]
Chemical properties
Extrapolated chemical properties
Certain chemical properties, such as bond lengths, are predicted to differ from what one would expect based on periodic trends from the lighter halogens (because of relativistic effects). It may have some metalloid properties, similar to astatine.[15]
References
- ↑ J. Chatt (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51: 381–384. doi:10.1351/pac197951020381.
- ↑ Element 117 discovered at physicstoday.org
- ↑ 3.0 3.1 3.2 Recommendations: 31st meeting, PAC for Nuclear Physics
- ↑ Yu. Ts. Oganessian et al., Synthesis of a New Element with Atomic Number Z=117, Phys. Rev. Lett. 104, 142502 (2010). DOI: 10.1103/PhysRevLett.104.142502.
- ↑ Koppenol, W. H. (2002). "Naming of new elements (IUPAC Recommendations 2002)". Pure and Applied Chemistry 74: 787. doi:10.1351/pac200274050787. http://media.iupac.org/publications/pac/2002/pdf/7405x0787.pdf.
- ↑ "Toward element 117 - CED - TASCA 08" (PDF). http://www-win.gsi.de/tasca08/contributions/TASCA08_Cont_Duellmann2b.pdf. Retrieved 2010-04-12.
- ↑ Ununseptium – the 117th element at AtomInfo.ru
- ↑ 8.0 8.1 Roman Sagaidak. "Experiment setting on synthesis of superheavy nuclei in fusion-evaporation reactions. Preparation to synthesis of new element with Z=117". http://159.93.28.88/linkc/education/SHE_Sagaidak.pdf. Retrieved 2009-07-07.
- ↑ Walter Grenier: Recommendations, a PowerPoint presentation at the January 2010 meeting of the PAC for Nuclear Physics
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 Zhao-Qing, Feng; Gen-Ming, Jin; Ming-Hui, Huang; Zai-Guo, Gan; Nan, Wang; Jun-Qing, Li (2007). "Possible Way to Synthesize Superheavy Element Z = 117". Chinese Physics Letters 24: 2551. doi:10.1088/0256-307X/24/9/024. http://arxiv.org/pdf/0708.0159.
- ↑ 11.0 11.1 Feng, Z; Jin, G; Li, J; Scheid, W (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A 816: 33. doi:10.1016/j.nuclphysa.2008.11.003. http://arxiv.org/pdf/0803.1117.
- ↑ C. Samanta, P. Roy Chowdhury and D.N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142. doi:10.1016/j.nuclphysa.2007.04.001.
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C 77: 044603. doi:10.1103/PhysRevC.77.044603.
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". At. Data & Nucl. Data Tables 94: 781–806. doi:10.1016/j.adt.2008.01.003.
- ↑ Trond Saue. "Principles and Applications of Relativistic Molecular Calculations". http://dirac.chem.sdu.dk/thesis/96.saue_phd.pdf., page 76
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||