Rhodium
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
silvery white metallic |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | rhodium, Rh, 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈroʊdiəm/ ROH-dee-əm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 9, 5, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 102.90550g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 5s1 4d8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 16, 1 (Image) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 12.41 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 10.7 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2237 K, 1964 °C, 3567 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3968 K, 3695 °C, 6683 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 26.59 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 494 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 24.98 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 6, 5, 4, 3, 2, 1[1], -1 (amphoteric oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.28 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 719.7 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1740 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2997 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 134 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 142±7 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[2] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 43.3 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 150 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 8.2 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 4700 m/s | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 380 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 150 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 275 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 1246 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 1100 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-16-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of rhodium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Rhodium (pronounced /ˈroʊdiəm/ ROH-dee-əm) is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. Naturally-occurring rhodium is composed of only one isotope, 103Rh. It is one of the rarest precious metals and, with a price of about US$80,000/kg in 2010, is the most expensive member of that class.[3]
Rhodium was discovered in 1803 by William Hyde Wollaston. It is found in platinum- or nickel ores together with the other members of the platinum group metals. Rhodium is mostly used as a catalyst in the three-way catalytic converter. Rhodium is inert against corrosion and most aggressive chemicals, and because of that and its rarity, rhodium is usually alloyed with platinum or palladium and applied in high-temperature and corrosion-resistive coatings. White gold is often plated with a thin rhodium layer to improve its optical impression while sterling silver is often rhodium plated for tarnish resistance. Rhodium detectors are used in nuclear reactors to measure the neutron flux level.
Contents |
History

Rhodium (Greek rhodon (ῥόδον) meaning "rose") was discovered in 1803 by William Hyde Wollaston,[4][5] soon after his discovery of palladium.[6][7] He used crude platinum ore presumably obtained from South America.[8] His procedure involved dissolving the ore in aqua regia and neutralizing the acid with sodium hydroxide (NaOH). He then precipitated the platinum by adding ammonium chloride, NH4Cl, as ammonium chloroplatinate. Most other metals like copper, lead, palladium and rhodium were precipitated with zinc. Diluted nitric acid dissolved all but palladium and rhodium, which were dissolved in aqua regia, and the rhodium was precipitated by the addition of sodium chloride as Na3[RhCl6]·nH2O. After washing with ethanol, the rose red precipitate was reacted with zinc forming rhodium metal.[9]
After the discovery the rare element had only minor applications, for example by the turn of the century rhodium containing thermocouples where used to measure temperatures up to 1800°C.[10][11] The first major application was electroplating for decorative uses and as corrosion resistant coating.[12] The introduction of the three way catalytic converter by Volvo in 1976 increased the demand for rhodium. The previous catalytic converters used platinum or palladium while the three way catalytic converter used rhodium to reduce the amount of NOx in the exhaust.[13][14][15]
Characteristics
Rhodium is a hard, silvery, durable metal that has a high reflectance. Rhodium metal does not normally form an oxide, even when heated.[16] Oxygen is absorbed from the atmosphere only at the melting point of rhodium, but is released on solidification.[17] Rhodium has both a higher melting point and lower density than platinum. It is not attacked by most acids: it is completely insoluble in nitric acid and dissolves slightly in aqua regia.
Chemical properties
Rhodium belongs to group 9 of the periodic table but has an atypical configuration in its outermost electron shells compared to the rest of the members. This can also be observed in the neighborhood of niobium (41), ruthenium (44), rhodium (45), and palladium (46).
| Z | Element | No. of electrons/shell |
|---|---|---|
| 27 | cobalt | 2, 8, 15, 2 |
| 45 | rhodium | 2, 8, 18, 16, 1 |
| 77 | iridium | 2, 8, 18, 32, 15, 2 |
| 109 | meitnerium | 2, 8, 18, 32, 32, 15, 2 |
| Oxidation states of rhodium |
|
|---|---|
| +0 | Rh4(CO)12 |
| +1 | RhCl(PH3)2 |
| +2 | Rh2(O2CCH3)4 |
| +3 | RhCl3, Rh2O3 |
| +4 | RhF4, RhO2 |
| +5 | RhF5, Sr3LiRhO6 |
| +6 | RhF6 |
Common oxidation states of rhodium is +3, but oxidation states from +0 to +6 are also observed.[18]
Unlike ruthenium and osmium, rhodium forms no volatile oxygen compounds. The known stable oxides include Rh2O3, RhO2, RhO2·xH2O, Na2RhO3, Sr3LiRhO6 and Sr3NaRhO6.[19] Halogen compounds are known in nearly the full range of possible oxidation states. Rhodium(III) chloride, rhodium(IV) fluoride, rhodium(V) fluoride and rhodium(VI) fluoride are some examples. The lower oxidation states are only stable if ligands are present.[20]
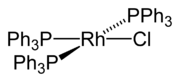
The best-known rhodium-halogen compound is the Wilkinson's catalyst chlorotris(triphenylphosphine)rhodium(I). This catalyst is used, for example, in the hydroformylation or hydrogenation of alkenes.[21]
Isotopes
Naturally-occurring rhodium is composed of only one isotope, 103Rh. The most stable radioisotopes are 101Rh with a half-life of 3.3 years, 102Rh with a half-life of 207 days, 102mRh with a half-life of 2.9 years, and 99Rh with a half-life of 16.1 days. Twenty other radioisotopes have been characterized with atomic weights ranging from 92.926 u (93Rh) to 116.925 u (117Rh). Most of these have half-lives shorter than an hour, except 100Rh (half-life: 20.8 hours) and 105Rh (half-life: 35.36 hours). There are also numerous meta states, the most stable being 102mRh (0.141 MeV) with a half-life of about 2.9 years and 101mRh (0.157 MeV) with a half-life of 4.34 days. (See isotopes of rhodium).[22]
The primary decay mode before the only stable isotope, 103Rh, is electron capture and the primary mode after is beta emission. The primary decay product before 103Rh is ruthenium and the primary product after is palladium.[23]
Occurrence
Normal mining
The industrial extraction of rhodium is complex as the metal occurs in ores mixed with other metals such as palladium, silver, platinum, and gold. It is found in platinum ores and extracted as a white inert metal which is very difficult to fuse. Principal sources are located in South Africa, in river sands of the Ural Mountains, and in North America, including the copper-nickel sulfide mining area of the Sudbury, Ontario region. Although the quantity at Sudbury is very small, the large amount of processed nickel ore makes rhodium recovery cost effective. The main exporter of rhodium is South Africa (>80%) followed by Russia.[24] The annual world production of this element is only about 25 tonnes and there are very few rhodium-bearing minerals. As of October 2007[update], rhodium cost approximately eight times more than gold, 450 times more than silver, and 27,250 times more than copper by weight. Rhodium's typical historical price is about $1,000/troy oz,[25] but in recent years, it has increased to about $4500/troy oz.[3]
In 2008, the price briefly rose above $10,000 per ounce.[3] The economic slowdown of the 3rd quarter of 2008 pushed rhodium prices sharply back below $1,000 per ounce, but they rebounced to $2,750 by early 2010 (over twice the gold price).[3]
From used nuclear fuels
Rhodium is a fission product of uranium-235; therefore, each kilogram of fission products contains significant amounts of the lighter platinum group metals including rhodium. Used nuclear fuel might be a possible source for rhodium. However, the extraction is complex and expensive, and the also present radioactive isotopes of rhodium would require a storage for several half-lives of the decaying isotopes in order to reduce the radioactivity. This makes this source of rhodium unattractive and no large-scale extraction has been attempted.[26][27][28]
Applications
The primary use of this element is in automobiles as a catalytic converter, which changes harmful emissions from the engine into less polluting gases.[29][30]
Catalyst

In 2007, 81%[29] of the world production of rhodium was consumed to produce three-way catalytic converters.[29] Rhodium shows some advantages over the other platinum metals in the reduction of nitrogen oxides to nitrogen and oxygen:[31]
- 2 NOx → x O2 + N2
The recycling of catalytic converters also became a valuable source for rhodium. In 2007, 5.7 t were extracted from this source. Compared to the 22 t which had been mined, this is a relatively high recycling rate.[29]
Rhodium-based catalysts are used in a number of industrial processes; notably, in the automobile catalytic converters and for catalytic carbonylation of methanol to produce acetic acid by the Monsanto process.[32] It is also used to catalyze addition of hydrosilanes to molecular double bonds, a process important in manufacture of certain silicone rubbers.[33] Rhodium catalysts are also used to reduce benzene to cyclohexane.[34]
The complex of a rhodium ion with BINAP gives a widely used chiral catalyst for chiral synthesis, as in the synthesis of menthol.[35]
Ornamental uses

Rhodium finds use in jewelry and for decorations. It is electroplated on white gold and platinum to give it a reflective white surface. This is known as rhodium flashing in the jewelry business. It may also be used in coating sterling silver in order to strengthen the metal against tarnish, which is silver sulfide (Ag2S) produced from the atmospheric hydrogen sulfide (H2S). Solid (pure) rhodium jewelry is very rare, because the metal has both high melting point and poor malleability (making such jewelry very hard to fabricate) rather than due to its high price.[36] Additionally, its high cost assures that most of its jewelry usage is in the form of tiny amounts of powder (commonly called rhodium sponge) dissolved into electroplating solutions.
Rhodium has also been used for honors, or to symbolize wealth, when more commonly used metals such as silver, gold or platinum are deemed insufficient. In 1979, the Guinness Book of World Records gave Paul McCartney a rhodium-plated disc for being history's all-time best-selling songwriter and recording artist.[37]
Other uses

Rhodium is used as an alloying agent for hardening and improving the corrosion resistance[16] of platinum and palladium. These alloys are used in furnace windings, bushings for glass fiber production, thermocouple elements, electrodes for aircraft spark plugs, and laboratory crucibles.[38] Other uses include:
- An electrical contact material due to its low electrical resistance, low and stable contact resistance, and high corrosion resistance.[39]
- Plated rhodium, made by electroplating or evaporation, is extremely hard and is used for optical instruments.[40]
- It is also used as a filter in mammography systems because of the characteristic X-rays it produces.[41]
- Rhodium is also used to coat some parts of high-quality pens due to its high chemical and mechanical resistance. These pens include Graf von Faber-Castell[42] and Caran D'ache.[43]
- Rhodium neutron detectors are used in Combustion Engineering Nuclear Reactors to measure neutron flux levels – a method that requires a digital filter to determine the current neutron flux level, as there are three signals generated: immediate, a few seconds later, and a minute later, each with its own signal level, and all three are combined in the rhodium detector signals. The three Palo Verde nuclear reactors each have 305 rhodium neutron detectors, 61 detectors on each of 5 vertical levels, providing an accurate 3-D "picture" of reactivity and allowing fine tuning to most economically burn the nuclear fuel.[44]
Precautions
Being a noble metal, pure rhodium is inert. However, chemical complexes of rhodium can be reactive. Median lethal dose (LD50) for rats is 12.6 mg of rhodium chloride (RhCl3) per kilogram of body weight.[45] Rhodium compounds can strongly stain human skin. The element plays no biological role in humans. If used in elemental form rather than as compounds, the metal is harmless.[46]
See also
- Rhodium compounds
- 2000s commodities boom
References
- ↑ "Rhodium: rhodium(I) fluoride compound data". OpenMOPAC.net. http://openmopac.net/data_normal/rhfr_jmol.html. Retrieved 2007-12-10.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ 3.0 3.1 3.2 3.3 "Rhodium.". www.kitco.com. http://www.kitco.com/charts/rhodium.html. Retrieved June 9, 2010.
- ↑ "WebElements – The History of Rhodium". webelements. http://www.webelements.com/webelements/elements/text/Rh/hist.html. Retrieved 2009-02-06.
- ↑ Wollaston, W. H. (1805). "On the Discovery of Palladium; With Observations on Other Substances Found with Platina". Philosophical Transactions of the Royal Society of London 95: 316–330. doi:10.1098/rstl.1805.0024.
- ↑ W. P. Griffith (2003). "Rhodium and Palladium – Events Surrounding Its Discovery". Platinum Metals Review 47 (4): 175–183. http://www.platinummetalsreview.com/dynamic/article/view/47-4-175-183.
- ↑ Wollaston, W. H. (1804). "On a New Metal, Found in Crude Platina". Philosophical Transactions of the Royal Society of London 94: 419–430. doi:10.1098/rstl.1804.0019.
- ↑ Lide, David R (2004). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. Boca Raton: CRC Press. pp. 4–26. ISBN 0-8493-0485-7.
- ↑ Griffith, W. P. (2003). "Bicentenary of Four Platinum Group Metals: Osmium and iridium – events surrounding their discoveries". Platinum Metals Review 47 (4): 175–183.
- ↑ Hulett, G. A.; Berger, H. W. (1904). Journal of the American Chemical Society 26: 1512. doi:10.1021/ja02001a012.
- ↑ Measurement, Astm Committee E.2.0. on Temperature (1993). "Platinum Type". Manual on the use of thermocouples in temperature measurement. ASTM International. ISBN 9780803114661. http://books.google.com/?id=Pos-MXDWb6MC&pg=PA63.
- ↑ Kushner, Joseph B. (1940). "Modern rhodium plating". Metals and Alloys 11: 137–140.
- ↑ Amatayakul, W (2001). "Life cycle assessment of a catalytic converter for passenger cars". Journal of Cleaner Production 9: 395. doi:10.1016/S0959-6526(00)00082-2.
- ↑ Heck, R (2001). "Automobile exhaust catalysts". Applied Catalysis A: General 221: 443. doi:10.1016/S0926-860X(01)00818-3.
- ↑ Heck, R (2001). "The application of monoliths for gas phase catalytic reactions". Chemical Engineering Journal 82: 149. doi:10.1016/S1385-8947(00)00365-X.
- ↑ 16.0 16.1 Cramer, Stephen; S., Jr Covino, Bernard (1990). ASM handbook. Materials Park, OH: ASM International. pp. 393–396. ISBN 0-87170-707-1. http://books.google.com/?id=QV0sWU2qF5oC&pg=PA396.
- ↑ Emsley, John (2001). Nature's Building Blocks ((Hardcover, First Edition) ed.). Oxford University Press. pp. 363. ISBN 0198503407.
- ↑ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1056–1057. ISBN 3-11-007511-3.
- ↑ Reisner, B. A.; Stacy, A. M. (1998). "Sr3ARhO6(A = Li, Na): Crystallization of a Rhodium(V) Oxide from Molten Hydroxide". Of the American Chemical Society 120: 9682–9989. doi:10.1021/ja974231q.
- ↑ Griffith, W. P. The Rarer Platinum Metals; John Wiley and Sons: New York, 1976; p 313.
- ↑ Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G. (1966). "The Preparation and Properties of Tris(triphenylphosphine)halogenorhodium(I) and Some Reactions Thereof Including Catalytic Homogeneous Hydrogenation of Olefins and Acetylenes and Their Derivatives". Journal of the Chemical Society A: 1711–1732. doi:10.1039/J19660001711.
- ↑ Audi, G. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ David R. Lide (ed.), Norman E. Holden in CRC Handbook of Chemistry and Physics, 85th Edition CRC Press. Boca Raton, Florida (2005). Section 11, Table of the Isotopes.
- ↑ Chevalier, Patrick (?). "Mineral Yearbook: Platinum Group Metals". Natural Resources Canada. http://www.nrcan-rncan.gc.ca/mms-smm/busi-indu/cmy-amc/content/2004/71.pdf. Retrieved 2008-10-17.
- ↑ Lide, D. R., ed. (2005), CRC Handbook of Chemistry and Physics (86th ed.), Boca Raton (FL): CRC Press, ISBN 0-8493-0486-5
- ↑ Kolarik, Zdenek; Renard, Edouard V. (2005). "Potential Applications of Fission Platinoids in Industry". Platinum Metals Review 49: 79. doi:10.1595/147106705X35263. http://www.platinummetalsreview.com/pdf/79-90-pmr-apr05.pdf.
- ↑ Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part I PART I: General Considerations and Basic Chemistry". Platinum Metals Review 47 (2): 74–87. http://www.platinummetalsreview.com/pdf/pmr-v47-i2-074-087.pdf.
- ↑ Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part II: Separation Process". Platinum Metals Review 47 (2): 123–131. http://www.platinummetalsreview.com/pdf/pmr-v47-i2-074-087.pdf.
- ↑ 29.0 29.1 29.2 29.3 George, Micheal W.. "Commodity Report: Platinum-Group Metals". United States Geological Survey USGS. http://minerals.usgs.gov/minerals/pubs/commodity/platinum/mcs-2008-plati.pdf. Retrieved 2008-09-16.
- ↑ George, Micheal W.. "2006 Minerals Yearbook: Platinum-Group Metals". United States Geological Survey USGS. http://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2006-plati.pdf. Retrieved 2008-09-16.
- ↑ Shelef, M.; Graham, G. W. (1994). "Why Rhodium in Automotive Three-Way Catalysts?". Catalysis Reviews 36 (3): 433–457. doi:10.1080/01614949408009468.
- ↑ Roth, James F. (1975). "Rhodium Catalysed Carbonylation of Methanol" (PDF). Platinum Metals Review 19 (1 January): 12–14. http://www.platinummetalsreview.com/pdf/pmr-v19-i1-012-014.pdf.
- ↑ Heidingsfeldova, M. and Capka, M. (2003). "Rhodium complexes as catalysts for hydrosilylation crosslinking of silicone rubber". Journal of Applied Polymer Science 30: 1837. doi:10.1002/app.1985.070300505.
- ↑ Halligudi, S. B. et al. (1992). "Hydrogenation of benzene to cyclohexane catalyzed by rhodium(I) complex supported on montmorillonite clay". Reaction Kinetics and Catalysis Letters 48: 547. doi:10.1007/BF02162706.
- ↑ Akutagawa, S. (1995). "Asymmetric synthesis by metal BINAP catalysts". Applied Catalysis A 128: 171. doi:10.1016/0926-860X(95)00097-6.
- ↑ Fischer, Torkel; Fregert, S; Gruvberger, B; Rystedt, I (1984). "Contact sensitivity to nickel in white gold". Contact Dermatitis 10 (1): 23–24. doi:10.1111/j.1600-0536.1984.tb00056.x. PMID 6705515.
- ↑ "Hit & Run: Ring the changes". London: The Independent. 2008-12-02. http://www.independent.co.uk/news/people/hit-and-run/hit--run-ring-the-changes-1044166.html. Retrieved 2009-06-06.
- ↑ Lide, David R (2004). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. Boca Raton: CRC Press. pp. 4–26. ISBN 0-8493-0485-7. http://books.google.com/?id=WDll8hA006AC.
- ↑ Weisberg, Alfred M. (1999). "Rhodium plating". Metal Finishing 97 (1): 296–299. doi:10.1016/S0026-0576(00)83088-3.
- ↑ Smith, Warren J. (2007). "Reflectors". Modern optical engineering: the design of optical systems. McGraw-Hill. pp. 247–248. ISBN 9780071476874. http://books.google.com/?id=DrtM_bAnf_YC.
- ↑ McDonagh, C P et al. (1984). "Optimum x-ray spectra for mammography: choice of K-edge filters for tungsten anode tubes". Phys. Med. Biol. 29: 249. doi:10.1088/0031-9155/29/3/004.
- ↑ "Guilloche luxury pen range by Graf von Faber-Castell". pensfromheaven. http://www.pensfromheaven.com/pens_luxury_Graf-von-Faber-Castell_Guilloche.htm. Retrieved 2010-06-04.
- ↑ "Caran D'Ache Ecridor Type 55 Rhodium Fountain Pen". Pengallery. http://www.pengallery.com/details.aspx?productID=2990. Retrieved 2010-06-09.
- ↑ Sokolov Pochivalin, G. P.; Shipovskikh, Yu. M.; Garusov, Yu. V.; Chernikov O. G.; Shevchenko V. G., A. P.; Pochivalin, G. P.; Shipovskikh, Yu. M.; Garusov, Yu. V.; Chernikov, O. G.; Shevchenko, V. G. (1993). "Rhodium self-powered detector for monitoring neutron fluence, energy production, and isotopic composition of fuel". Atomic Energy 74: 365–367. doi:10.1007/BF00844622.
- ↑ Landolt, Robert R.; Berk Harold W.; Russell, Henry T. (1972). "Studies on the toxicity of rhodium trichloride in rats and rabbits". Toxicology and Applied Pharmacology 21 (4): 589–590. doi:10.1016/0041-008X(72)90016-6. PMID 5047055.
- ↑ Leikin, Jerrold B.; Paloucek Frank P. (2008). Poisoning and Toxicology Handbook. Informa Health Care. pp. 846. ISBN 9781420044799. http://books.google.com/?id=0Bw2UJTC_uMC.
External links
- WebElements.com – Rhodium
- Current Rhodium price
- Rhodium Technical and Safety Data
- Los Alamos National Laboratory – Rhodium
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||